TRANSFORMATION HAEMOGLOBIN IN UREA - CREATININ
INTRODUCTION
This work is an untraditional look at the chemistry content, of two materials in blood.
These materials (Urea-Creatinin) increase in blood if kidney disease is present.
Urea-Creatinin materials are the product of Haemoglobin transformations, and the proof of this is mutual relation of Haemoglobin molecules and Urea - Creatinin molecules.
This is a new point of view, and offers a new possibility for Oxygen and vitamin treatment of Kidney diseases.
This is the explanation of chemistry content,and the explanation creations of Urea-Creatinin.
Four theses are connected with-Kidney disease, and intended for doctors.
The theses are theoretical, illustrated, and awaiting acknowledgment.
Hypothesis 1
Urea and Creatinin evolve in the blood as a result of the transformation of Haemoglobin, and exchange the Iron (Fe) core of haemoglobin with a Carbon (C) atom from CO2 creating the core of Urea - Creatinin.
Hypothesis 2
Urea and Creatinin are the same substance. Urea and Creatinin have the same basic form. That basic form can change structure in both directions: - from Urea in Creatinin and from Creatinin in Urea.
Hypothesis 3
Urea and Creatinin have a basic form, which can disintegrate with the same method.
Hypothesis 4
Natural disintegration of Urea-Creatinin in carbon dioxide and water can explain: Anaemia, Lung-Kidney and Leukaemia diseases.
THESIS 1
Simple analysis
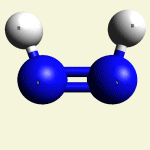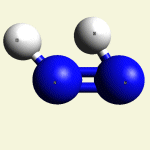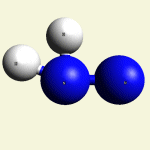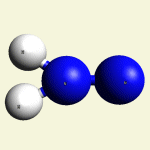
| Urea (NH2)2CO | |
| Solid atoms consist of: | Gas atoms consist of : |
| - 1 Carbon atom | - 2 Nitrogen atoms |
| - 4 Hydrogen atoms | |
| - 1 Oxygen atom | |
| Creatinin C4H7N3O | |
| Solid atoms consist of: | Gas atoms consist of : |
| - 4 Carbon atoms | - 3 Nitrogen atoms |
| - 7 Hydrogen atoms | |
| - 1 Oxygen atom | |
Graphic analysis
Urea (NH2)2CO Creatinin (C4H7N3O)
Creation of Urea
(NH2)2CO
Haemoglobin disease deforms the structure of haemoglobin, and a spiral substance
separates Iron (Fe) core from Oxygen (O2) atoms. The transport of Oxygen decreases,
and Oxygen is missing in the blood and in cells of the body.
The body cells release CO2 (carbon dioxide) that generates hydrocarbon CH4
(methane)in 2H2O (water).
Basic formula:
| CO2 + 2H2O → CO2 + H4O2→ CH4 + O2 + O2 |
Two Oxygen atoms are transferred in the body cells, and these two Oxygen atoms connect with hydrocarbons.
| CH4 + O2→ CH4O2 |
Four Nitrogen (N) atoms and the connection with the Iron (Fe) core of haemoglobin disintegrate. The Iron (Fe)
core excludes from Haemoglobin and Carbon (C) atom from CO2 is attracted in the structure.
(See Iron (Fe) core of Haemoglobin)
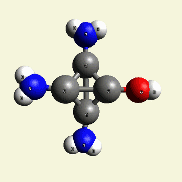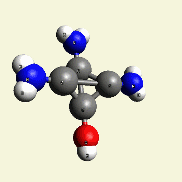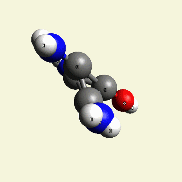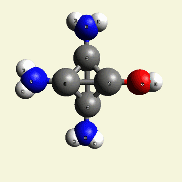
A pair of Nitrogen atoms connect with CH4O2 (hydrocarbon dioxide) and constitute Urea. (Figure 1)
| N2 + CH4O2→ (NH2)2CO |
One Oxygen (O) atom connects to a molecule in the urea structure chain, and a second Oxygen atom
is excluded.
| Figure 1 | 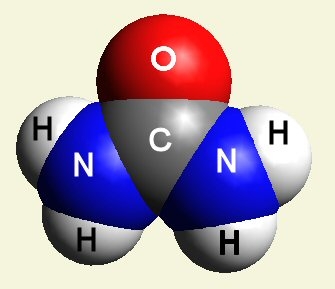 |
| O2 + C → CO2 |
Now a chain reaction begins and the process repeats:
| CO2 + 2H2O → CH4 + O2 + O2 |
| CH4 + O 2 + O 2 → in body cells |
↓ |
| CH4 + O 2 |
| Creations with a two Urea structure are possible with connection of four Nitrogen(N) atoms, from one transformable Haemoglobin cell. |
One Haemoglobin molecule is destroyed, and two Urea or a half Creatinin structure are created.
| N2 + CH4O2 creates a second Urea molecule. |
| Figure 2 |  |
 |
Oxygen (O2) transports Carbon (C) atoms from the body cells, and with a 2H2O connection a chain reaction of
CO2 + 2H2O continues.
| increase Urea - Creatinin value = decrease in Haemoglobin value |
Creation of Creatinin (C4H7N3O)
A four Urea structure is created and two Haemoglobin molecules are destroyed. A stable
structure has a basic
Creatinin form (figure 3), with surplus of 7 Oxygen, 9 Hydrogen and 5 Nitrogen atoms.
| Figure 3 | 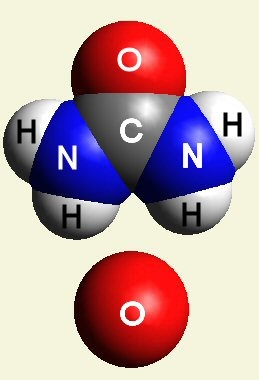 |
 |
 |
 |
A Carbon atom core is in the connection with Nitrogen (N) atoms and four Hydrogen (H) atoms.
Two pairs of Nitrogen atoms form the chain.
The structure of Creatinin is created, but the structure is not compact because it
contains different physical properties (Carbon is solid , Nitrogen is gas , Hydrogen and
Oxygen is gas or liquid).
Therefore four Carbon (C) atoms form the core in Creatinin molecule.
Hydrogen, Nitrogen and Oxygen atoms now transform the gas chain by attracting or
excluding atoms.
Creatinin molecule in Figure 4
| Figure 4 | 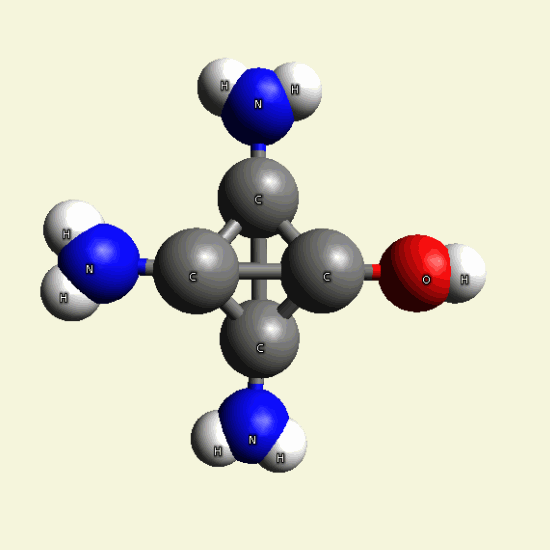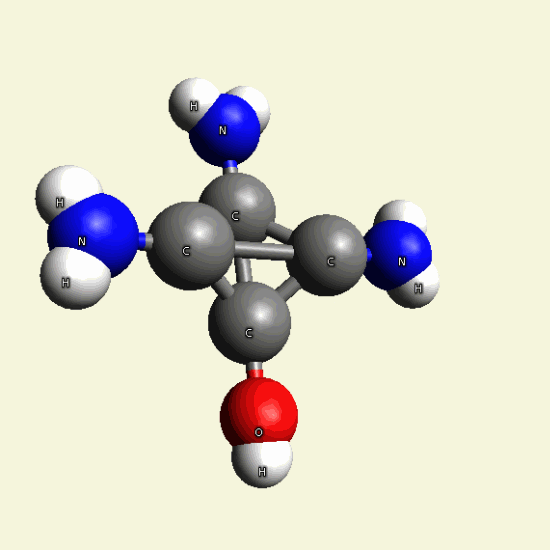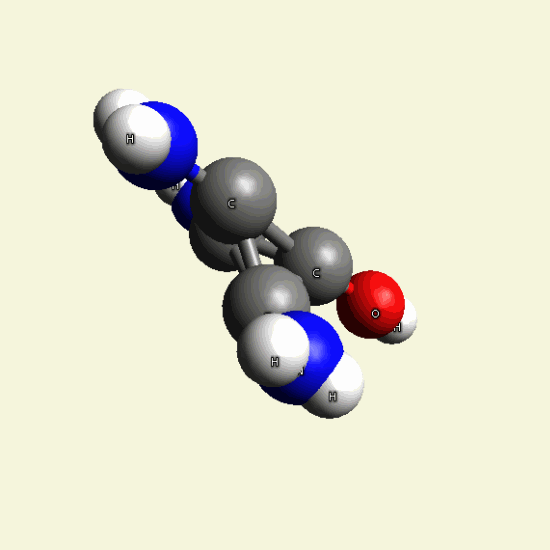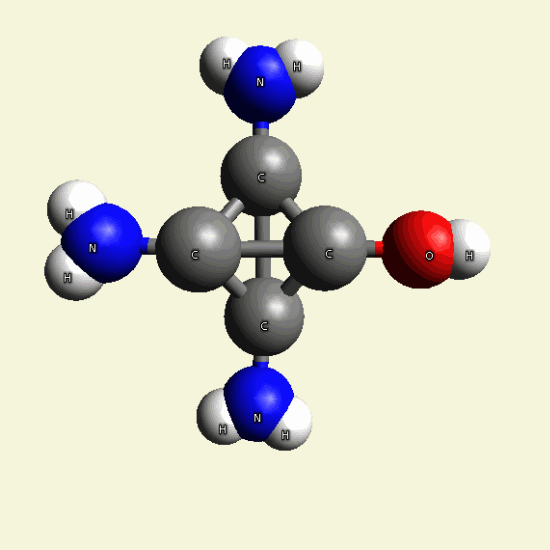 |
Simple control Creatinin structure
The composition of the Creatinin structure:(See Figure 3)
| Total Creatinin structure |
1.includes | excludes | missing | surplus | 2.includes | excludes | |
| 4 Carbon atoms | 4 | 4 | 4 | ||||
| 16 Hydrogen atoms |
7 | 9 | 7 | 2 | |||
| 8 Nitrogen atoms | 3 | 5 | 3 | 2 | |||
| 8 Oxygen atoms | 1 | 7 | 1 | 1 |
A chain reaction continues with 7 Oxygen atoms, and Oxygen connects with Carbon(C) from cells in the form of CO2.
The fourth Carbon (C) atom in the form of CO2 is transported by O2 from the body cells, and included in the second Creatinin structure.
The surplus of 1 Oxygen (O) atom is included in another Creatinin molecule.
Six Oxygen atoms are in the mutual relation with eight CO2 and four 2H2O cycles.
See Creatinin O2 cycle
Another Creatinin molecule excludes two Hydrogen and two Nitrogen atoms. See Figure 5
| Figure 5 |  |
This form is the beginning of the next Urea - Creatinin structure.
| For creating four Urea or two Creatinin structures, two Haemoglobin molecules are transformed. |
THESIS 2
UREA (NH2)2CO
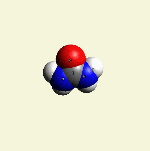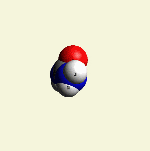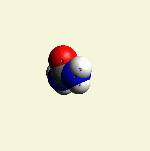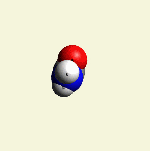
| 2H2O |
Both Urea and Creatinin have the same beginning; CO2 (carbon dioxide) + 2H2O (water).
Water content determines the direction of the chemistry process.
| From body cells ↓ 2H2O → H4O2 → H4 +(O2 +C) → H4+ CO2 → CH4O2 → |
| CH4O2 + H2N2 (structure)→ N2H4CO + H2O → (NH2)2CO excludes H2O |
| The process repeats with the surplus of H2O |
| H2O + CO2→H2CO3 + H2 N2 → N2H4CO + O2 →(NH2)2CO excludes O2 |
| Process repeats with the surplus of O2 (see Creatinin) |
| CONCLUSION: Urea has two creation modes. | |
| COMPLETE CREATION | HALF CREATION |
| 2*(CH4O2)+(N2)+(N2) | CH4O2 + H2N2 |
| One Haemoglobin molecule | H2CO3 + H2 N2 |
CREATININ C4H7N3O
| O2 |
Creatinin O2 cycle
Creation of the Creatinin is a repeating process, in the first 2x4 cycles Oxygen (O2) transfers Carbon (C) from
body cells into the two Creatinin structures :
1. O2+ C →(CO2)→ C atom is attracted into the structure, and O2 goes into the next cycle.
2. O2+ C →(CO2)→ C2 atoms are attracted into the structure, and O2 goes into the next cycle.
3. O2+ C →(CO2)→ C3 atoms are attracted into the structure, and O2 goes into the next cycle.
4. O2+ C →(CO2)→ C4 four carbon atoms form the core.
1. O2+ C →(CO2)→ C atom is attracted into the structure, and O2 goes into the next cycle.
2. O2+ C →(CO2)→ C2 atoms are attracted into the structure, and O2 goes into the next cycle.
3. O2+ C →(CO2)→ C3 atoms are attracted into the structure, and O2 goes into the next cycle.
4. O2+ C →(CO2)→ C4 four carbon atoms form the core.
In the next four cycles O2 connects Hydrogen (H) atoms from 2H2O with Creatinin.
1. O2+H4→(2H2O)→ H4atoms are attracted into the structure,and O2 goes into the next cycle.
2. O2+H4→(2H2O)→H8 atoms are attracted into the structure, and O2 goes into the next cycle.
3. O2+H4→(2H2O)→ H12atoms are attracted into the structure, and O2 goes into the next cycle.
4. O2+H4→(2H2O)→ H16 atoms are attracted into the structure, and O2 goes into the next cycle.
| In the two Creatinin structures there are eight Nitrogen (N) atoms from two transformed Haemoglobin molecules. |
| CONCLUSION | ATTRACTED | EXCLUDED |
| Cycle 1. is 2(C + H4 + N2) | ||
| Cycle 2. is 2(C + H4 + N2) | ||
| Cycle 3. is 2(C + H4 + N2) | ||
| Cycle 4. is 2(C + H4 + N2) | 2(C4H7N3O) | H2N2(structure) |
THESIS 1 and 2 |
| Graphic analysis |
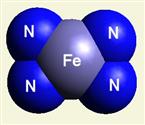
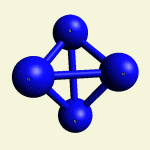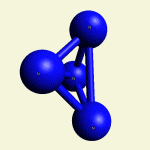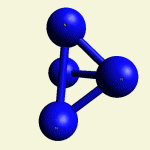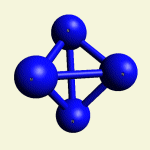
IRON (Fe) CORE OF HAEMOGLOBIN |
| Fe |
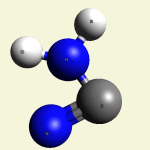
The Haemoglobin core consists of two pairs of Nitrogen (N) atoms connected to an Iron (Fe) atom in the centre
(See Figure 6)
| Figure 6 | Molecule content | |
 |
||
| FeN4 | ||
The valency of the Iron (Fe) is only two electrons in the 4. shell.
Carbon has four electrons in 2. shell , and a stable CO2 connection.
| The central atom changes place, and the Carbon (C) atom changes the structure of Haemoglobin. Valency defines the position of atoms, with the Carbon (C) atom in the centre. (Figure 7) |
| FeN4 + CO ( See CO in Example 14) → CN4 → CN2O excludes Fe excludes N2 |
See Iron
See Nitrogen
| FeN4 + CO + O ( See CO + O in Example 22B) → CN2O excludes FeN2O |
| FeN4 + CO2 ( See CO2 in Example 23) → CN2O2 excludes Fe excludes N2 |
| Figure 7 | 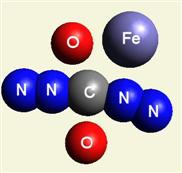 |
← Include C atom |
| → Exclude Fe atom |
Free Oxygen (O2) atoms in the next cycle transport four Hydrogen (H) atoms.
Hydrogen is transported with Oxygen in the form of 2H2O.
The Urea structure can include and exclude atoms (Figure 8)
| Figure 8 |  |
← Include H4 |
| → Exclude N2 |
| We have a closed cycle with (H2 N2) structure form.(Figure 9 and 4) |
| Figure 9 |  |
← Include H2O |
| → Exclude O |
| The remaining Oxygen (O) atom transports two Hydrogen (H) atoms in the form of H2O |
| Next step: H2 N2 + H2O + CO2 →(NH2)2CO →Exclude O2 |
| Next step: O2+ 2C4 →C8O2+H16+ N8 →2(C4 H7N3O)→Exclude H2 N2 |
| TRANSFORMATION OF HAEMOGLOBIN IN UREA CREATININ IS DEMONSTRATED |
THESIS 3 |
UREA - CREATININ DISINTEGRATION |
| Urea molecule (NH2)2CO disintegrates in three modes: | |
| Disintegrates in: | H2O (See Example 14) |
| CH4 (See Example 28) | |
| NH3 (See Example 29) | |
| CO (See Example 14) | |
| Urea molecule (NH2)2CO2 disintegrates in two modes: | |
| Disintegrates in: | 2H2O (See Example 30) |
| CH4 (See Example 28) | |
| CO2 (See Example 29) | |
Urea and Creatinin have the same basic form (CO2 +2H2O), and possibly for disintegration by the same method .
ENERGY (N) COMPONENT" |
| N 4 Nitrogen atoms from Urea - Creatinin can be removed with B6 Vitamin |
Vitamin B6 substance - Pyridoxine metabolises protein and amino acids.
Amino acids contain (N) Nitrogen atoms, and with B6 vitamin, amino acid converts into Protein. Protein gives muscle energy.
| Pyridoxine helps in removing the Nitrogen from amino acids, making them available as sources of muscle energy. |
CREATION OF AMINO ACID |
When Urea -Creatinin is disintegrated, all its chemical elements are included in the amino acid. Carbon atom is the core, with a Nitrogen connection in the atoms structure.
Amino acids contains many different elements.
UREA OXYGEN BALANCE
In the graphic below we see two graphic molecules together.
By breathing Oxygen O2 Urea disintegrates in: hydrogen peroxide H2O2 See Example
| N2 H4 C O N2 H4 C O |
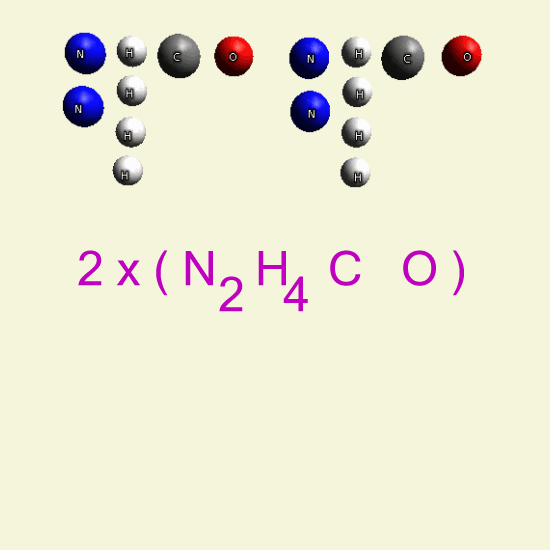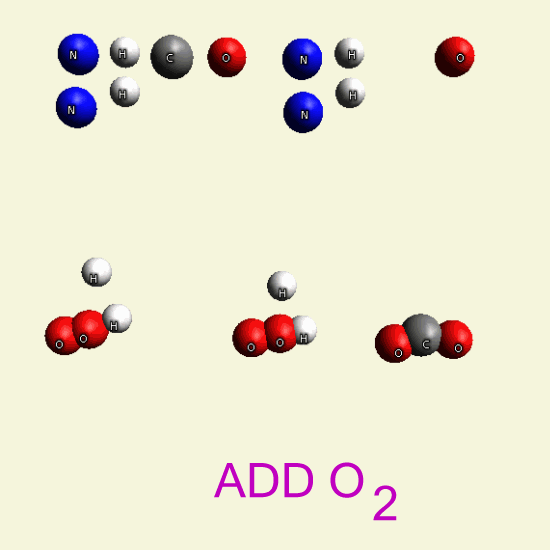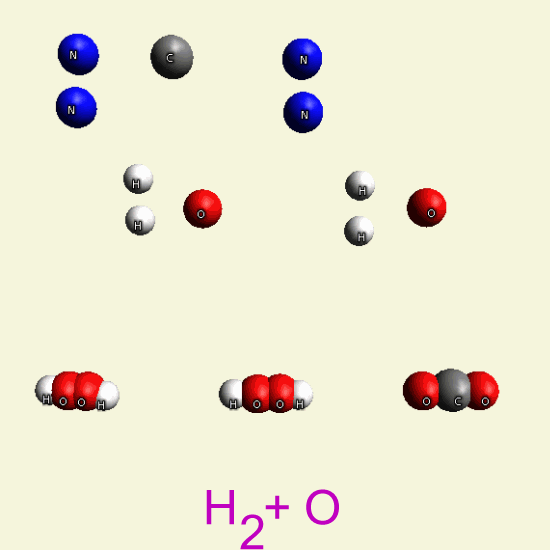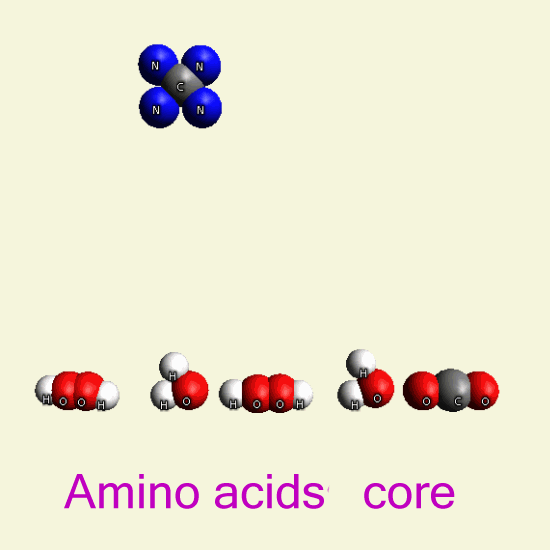
| H2+ O2 → H2 O2 → |
| H2O + O |
| C + O2 → CO2 |
By breathing Oxygen O2 Urea disintegrates from: hydro carbon CH4 See Example 28
| N2 H4 C O N2 H4 C O |
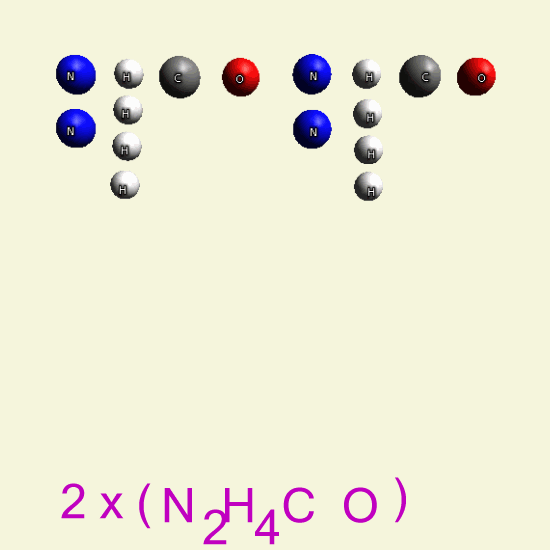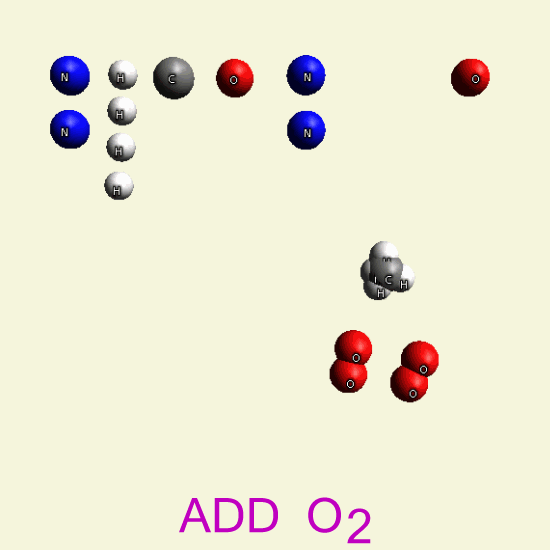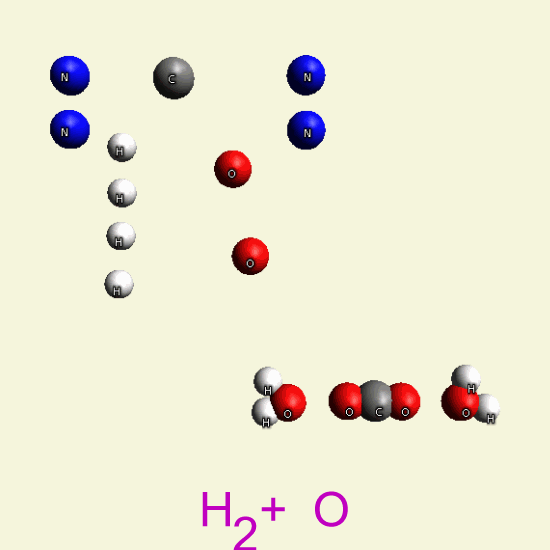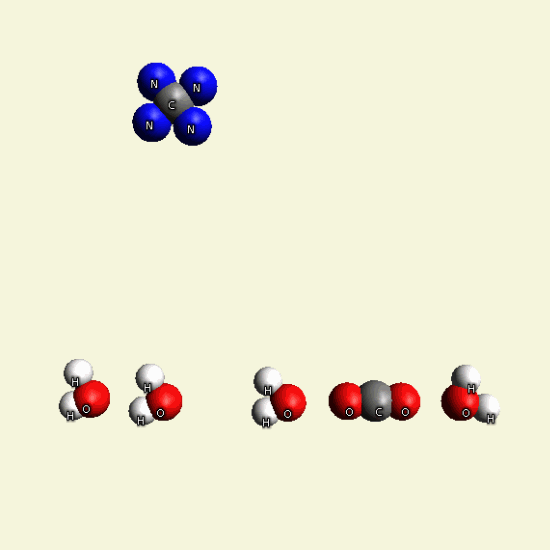
| C + H4 → CH4 + 2O2 → CO2 + 2H2O |
CREATININ OXYGEN BALANCE
In the graphic below we see two graphic molecules together.
By breathing Oxygen O2 disintegrate two Creatinin structures from hydrocarbon in: CH4, H2O, CO2, H2O2
| C4 H7 N3 O C4 H7 N3 O |
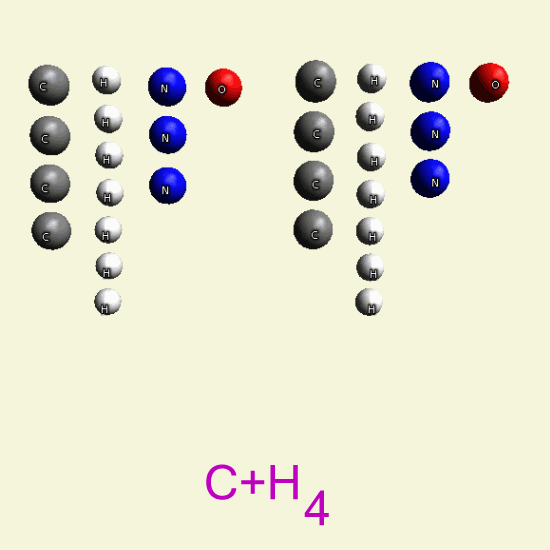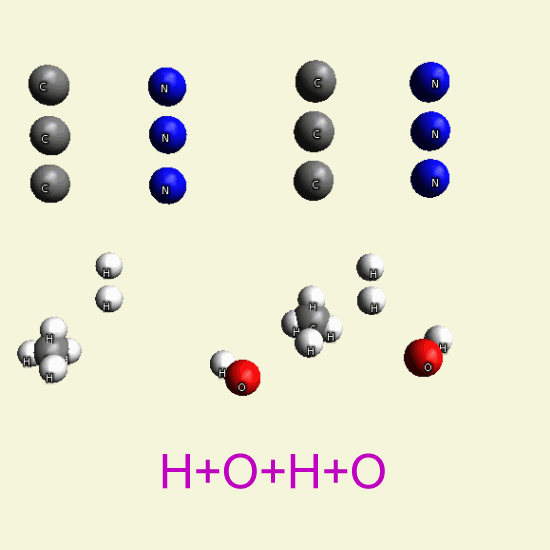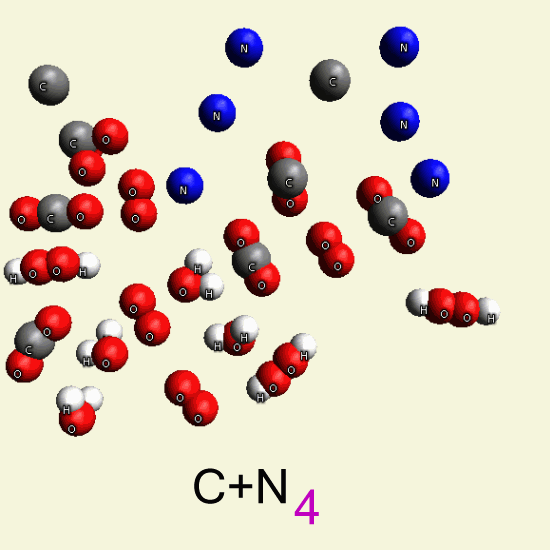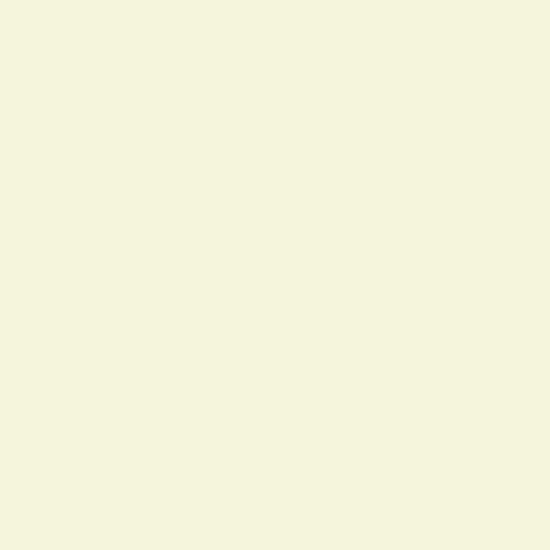
| C + H4 → CH4 + 2O2 → CO2 + 2H2O |
| C + O2 → CO2 |
| H + O + H + O → H2 O2 |
Balancing Creatinin is possible with Oxygen O2 atoms.
The result of disintegration is 4H2O (water molecules) and O2 Oxygen for the next disintegrated cycles.
Disintegrated Creatinin in: carbonic acid H2CO3
By breathing Oxygen (O2), the Creatinin structure disintegrates from H2CO3 in: CO2 and H2O, or CO and H2O2
| C4 H7 N3 O C4 H7 N3 O |
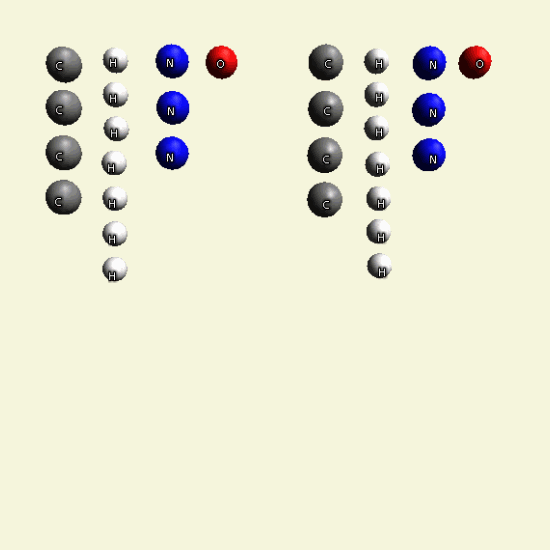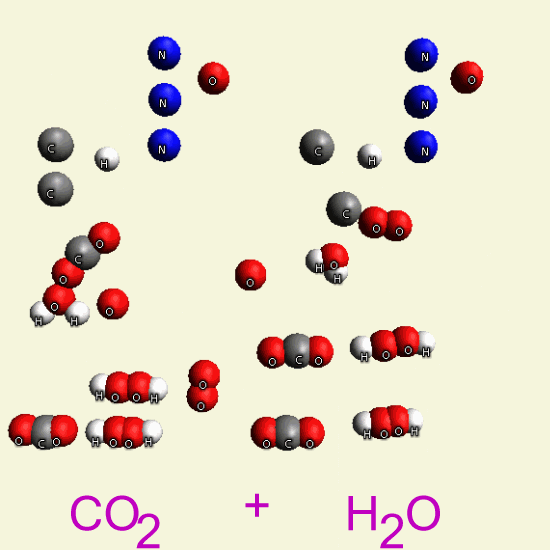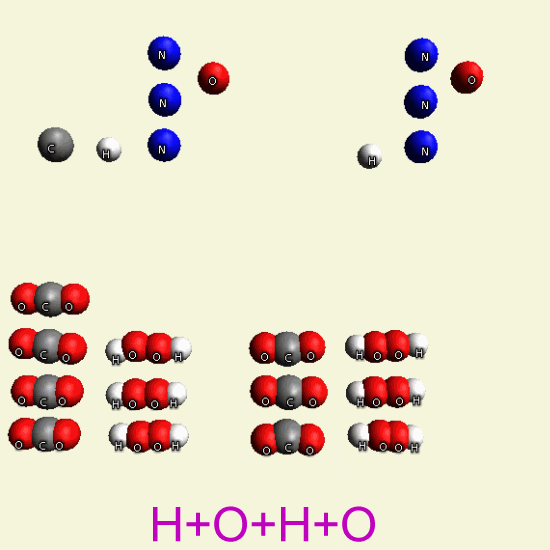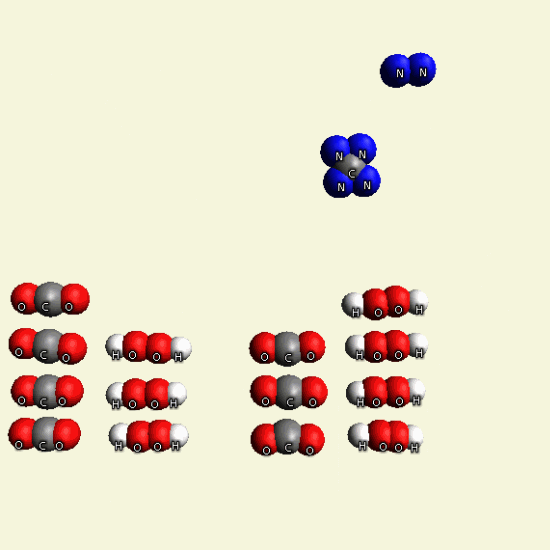
| CO2 + H2O → H2CO3 → |
| H2CO3 → CO2 + H2O |
| H2CO3 → CO + H2O2 |
Balancing Creatinin is possible with Oxygen (O2) atoms.
The result of disintegration is that CO2 and H2O are present in all combinations, and the creation of Urea Creatinin occurs continuosly.
The breathing of Oxygen 2O2 is important to prevent the initiation of chain reactions and Urea-Creatinin creation.
OXYGEN CONNECTION
| 2O2 |
Oxygen 2O2 is connect in the lungs with haemoglobin's Iron (Fe) core.
In the cells of the body 2O2 Oxygen disintegrates into O2 + O2. Oxygen for cell breathing connect Carbon from cells and in form CO2 is breathed out into the atmosphere.
One Oxygen O2 atoms go into the body cells, and a second Oxygen O2 atom connects with a Carbon (C) atom and leaves the body cells in the form of CO2. This is normal healthy 2O2 cycle.
Disease develops according to the CO2 % that remains in the blood after the lung gas exchange.
THESIS 4
CONCLUSION:
Urea-Creatinin in the blood is the result of a deficiency of Oxygen(O2) and transformation of Haemoglobin's
Iron(Fe) core in the Carbon(C) core of Urea-Creatinin. Carbon core Creatinin contains four Carbon atoms,
which accumulate in the Glomerulus,and prevent H2O water secretion.
Water accumulates in the blood, and the creation of Urea Creatinin begins in the reaction with Carbon dioxide.
Disintegration of Urea-Creatinin is the connection of Oxygen with all chemical elements,and the aim is
the natural breathing out of the (CO2) in the atmosfere and the kidneys secretion H2O.
Natural Urea-Creatinin disintegration has three forms:
ANAEMIA form |
The transformation from Haemoglobin in Urea - Creatinin is a slow process.
Disintegrated elements CO2 and H2O are naturally excreted by the lungs and in the kidneys.
LUNG and KIDNEY disease form |
The transformation from Haemoglobin in Urea-Creatinin is a slow process.
The lungs do not excrete the disintegrated element CO2, and carbon atoms increase in the blood and accumulate in the glomerulus. Secretion of H2O by the Kidneys decreases, and both CO2 and H2 O increase in the blood.
The initiation of a chain reaction CO2 + H2O is created.
Transformation from Haemoglobin in Urea Creatinin has a balanced form:
| 2 Haemoglobin molecules = 4 Urea molecules = 2 Creatinin molecules |
Oxygen and Iron insufficiency cause CO2 central atom changes.
Carbon atom is basic for the transformation of Haemoglobins in Urea
Creatinin, and can help in understanding Anaemia, Kidney disease and Leukaemia.
LEUKAEMIA form > |
The transformation from Haemoglobin in Urea Creatinin is individual.
Naturally disintegrated elements CO2 and H2O are naturally excreted by the lungs and kidneys.
Haemoglobin disappears and Urea Creatinin is not created.
A characteristic of Leukaemia is unbalanced Lung - Kidney function.
The Carbon atom from CO2 is removed, and free O2 connects with Hydrogen
atoms. The connection of Oxygen and Hydrogen atoms has H2O and 2H2O forms.
Nitrogen atoms from destroyed Haemoglobin cells do not create Urea Creatinin because of the missing Carbon (C) atom core (CO2 excreted by the lungs).
Low Oxygen and haemoglobin cause tiredness. The kidneys normally separates H2O and the disease can hide and diagnosis is difficult.
Author
I have a subconscious instinct to search for the cause of my disease. Despite ten years of treatment, the disease has not been cured.
My conclusion is that medical treatments are not effective because the disease
has progressed. I believe that by continually searching I must eventually achieve results.
Suddenly one night, my subconscious mind began to dictate this theoretical work.
All my arguments have the aim, discussions in doctor's circles and practical acknowledgment or excluding.
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Part two
SOLIDIFICATION PROCESS FOR CREATININE
The transformation from haemoglobin in urea-creatinin is a slow chemical process. When chemical transformation is finished, the creatinin molecule excludes H7N3O in one solidification process (Figure10).
The solidification process attracts central C4 carbon atoms to the centre of the molecule and excludes atoms from the periphery.
Creatinin molecule disintegrates in:
- stable carbon C4 molecule with a covalent bond between C atoms.See Carbon data
- two NH3 (ammonia) molecule see Ammonia data
- one H2O2 (hydrogen peroxide) see Hydrogen peroxide data (water molecule + oxygen atom)
- N2 (nitrogen atoms)
The excluded H7N3O atoms form two NH3 (ammonia) molecules,
and remain NHO-nitrogen, hydrogen and oxygen atoms attract NHO-nitrogen, hydrogen and oxygen atoms from another creatinin molecule (see two graphic molecules together).
N + N → N2
N2 can remain in the blood or can be excreted in urine.
For N2 in blood (See Creation of Urea (NH2)2CO)
and HO + HO → H2O2
H2O2 → H2O + O
(See Urea - Creatinin Disintegration)
Solidification process (see two graphic molecules together.Figure 10)
| Figure 10 | 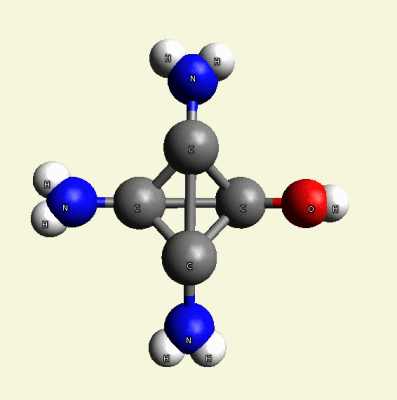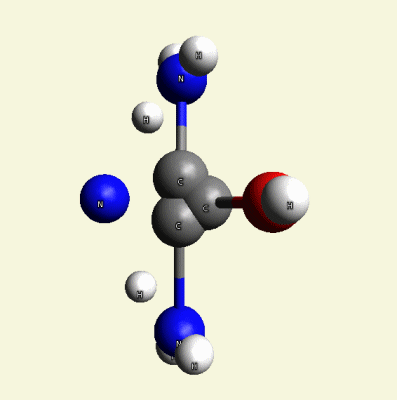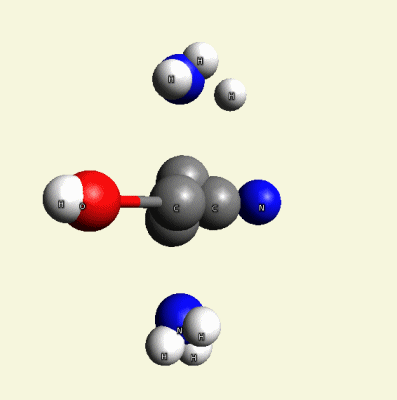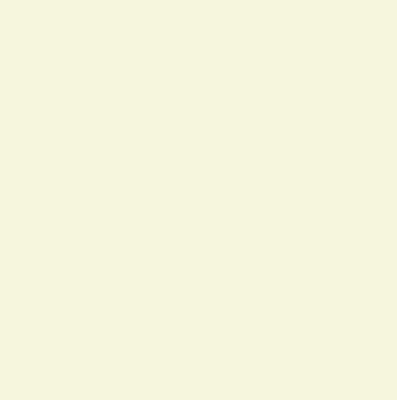
|
One creatinin molecule disintegrates in:
- stable carbon C4 molecule with a covalent bond between C atoms. See Carbon data
- one NH3 (ammonia) molecule see Ammonia data
- one H2O (water) molecule see Water
- N2H2 (nitrogen and hydrogen atoms) (see Figure 4)
Solidification process (one graphic molecule)(Figure11).
| Figure 11 | 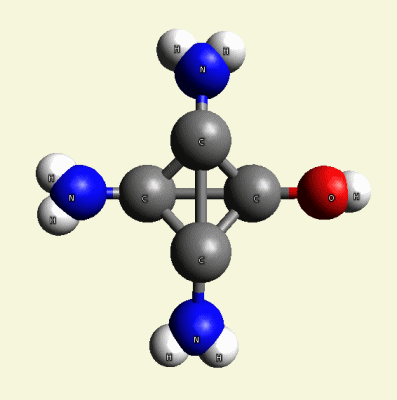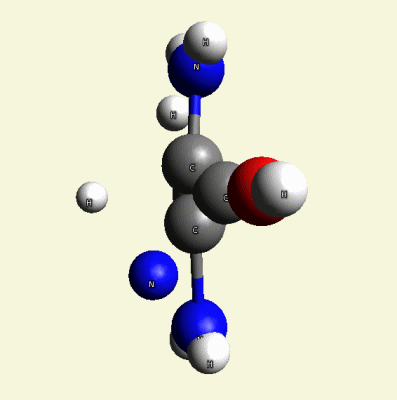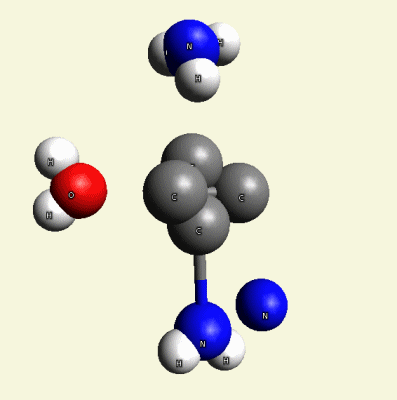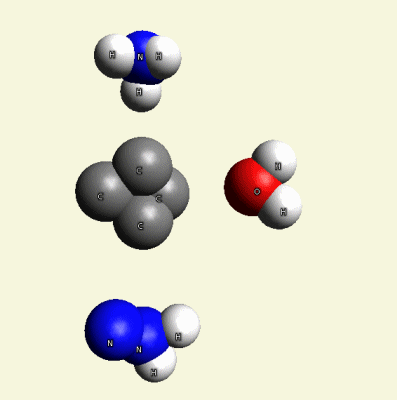
|
C4 CARBON NANO FILTRATION |
The body performs self regulation of water.
If oxygen in the blood is low, body cells obtain it from cell water (See Thesis 2)
, and blood has a high concentration of CO2 (carbon dioxide) See Carbon dioxide and CH4 (methane) See Methane .
This is very important for the separation of O2 (oxygen) and H2O (water) from CO2 (carbon dioxide) and CH4 (methane) gas molecules.
The kidneys' glomerulus separates chemical elements and accumulates carbon in capillaries.
Carbon atoms create a nanonet and include C (carbon) atoms and exclude remains. The remains of the chemistry elements pass or not pass across the nanonet.
The carbon atoms' nanonet includes C (carbon atom) from CO2 (carbon dioxide), and excludes O2 , two oxygen atoms see Oxygen.
From molecules, CH4 (methane) separates carbon, and excludes H4 hydrogen atoms.
Excluded O2 and H4 create O2+ H4 → 2H2O (Figure 12).
| Figure 12 | 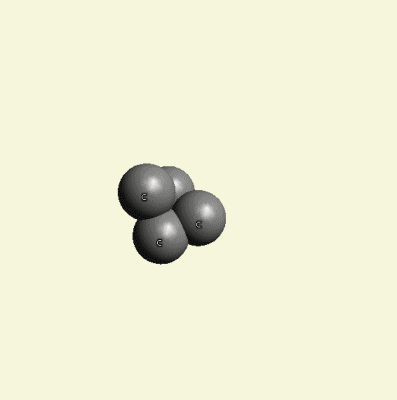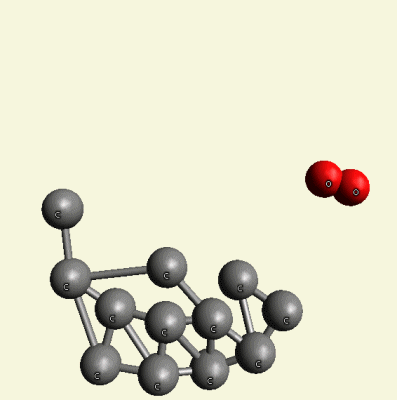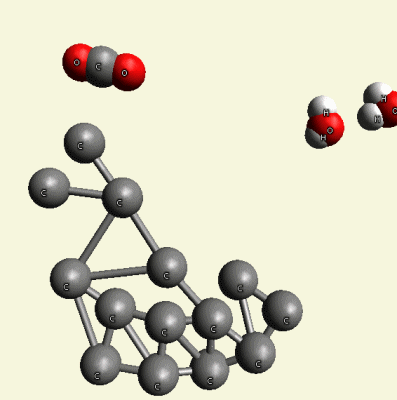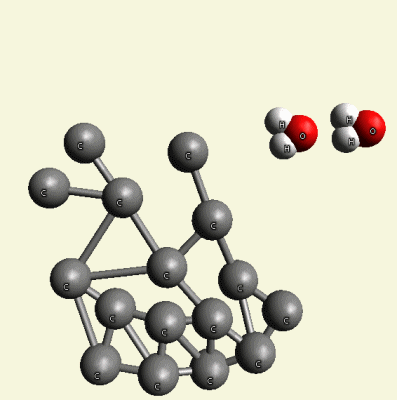
|
This water remains in the blood or passes across the carbon net.
The carbon nanonet enlarges volume and decreases flow in the capillaries, accumulating more and more excluded chemistry elements, finally creating a blood clot.
see ( g) Amorphous Carbon Nanonet
| Solidification process and chemistry transformation is finished. |
CELL HAEMOGLOBIN Creatinin Value
| Figure 13 |
The cell haemoglobin [C] value is the number (5), which indicates the (creatinin molecule) number.
Cell haemoglobin has 4 haemo with (Fe) iron atoms in the centre (Figure 13).
Calculated with 16 nitrogen atoms in 4 haemo as basic (Figure 14).
Cell haemoglobin has 16 N (nitrogen atoms). This is enough to create 5 creatinin molecules, and 1nitrogen atom remains as a surplus.
Two cells of haemoglobin are enough for 10 creatinin molecules and 1 urea molecule.
| Figure 14 | ||||
| 1 | 2 | 3 | 4 |
10 creatinin molecules and 1 urea molecule have 10 x 4 + 2 = 42 (C) carbon atoms see Carbon data..
Disintegration of two haemoglobin cells can create 42 carbon atoms and a nanonet clot. Carbon atoms accumulate as a cap in the glomerulus capillaries.
The C4 carbon molecule is stable and can connect with another carbon molecule chemically.
Components of urine are chemically excluded molecules end atoms; that is chemistry excludes elements and they cannot be connected.
Clots in the heart, brain and the kidneys clot form due to the different pressure.
All blood and urine molecules and elements are under the influence of:
- different pressure
- temperature
- speed flow
- flow resist
- viscosity
and time.
Those components have characteristics in the LAWS OF PHYSICS.
GLOMERULAR CLINING
LAWS OF PHYSICS
Each blood vessel has a different pressure, speed flow, flow resistance, and blood consistency. The pulse is a time characteristic.
Different pressures in the body are measured in (mmHg). The pulse is measured in beats per minute.
It is measured in mmHg for systolic blood pressure and diastolic pressure,and pulse measuring is separated.
Blood flow is a pulse component; flow resistance and consistency are pressure components and depend on temperature.
See Law of Physics for different pressure
See More In Renal Blood Flow
| Systolic blood pressure - Diastolic pressure = Flow resist |
see Vascular resistance
see Hypertension
Table 1
| Pulse/min | Blood amount [mil] | Systolic mmHg | Diastolic mmHg | Flow resist mmHg/d | Capilar mmHg | Bowman tub mmHg |
|---|---|---|---|---|---|---|
In flow resistance mmHg/d (d = diameter) (for exam... the diameter of the afferent arterioles is equal to the sum of the diameters of all glomerulus capillary vessels).
For example..(Table 1)
Pulse 50, blood amount 10 millilitres, blood volume is 500 mil/min (50x10=500).
If pulse increases, the volume also increases . If blood volume increases, systolic blood pressure increases too. Diastolic pressure and flow resistance increase flow speed and
decrease pressure inside the glomerular capillaries (Laws of Physic).Speed increasing maintains the blood amount, and the diastolic blood amount remains equal to the systolic blood amount. |
(see Law of Physics)
CONCLUSION:
Blood pressure inside the glomerulus capillaries is equal to the pressure in the Bowman´s capsule,
|
Each body has individual blood pressure and different blood vessels. There is a specific blood pressure ZONE (Figure 14.1) for precise blood pressure, for each organism. Medical blood pressure decreases or increases can move capillary filtration from the Bowman's capsule (Table 1).
If blood pressure decreases, capillary vessel pressure increases and creates a different pressure in the Bowman's capsule. This pressure is not equal to the pressure in the Bowman's tubule, and no filtration from the glomerulus occurs.
| Figure 14.1 |
Different pressure between blood inside capillary vessels (glomerulus) and urine outside capillary vessels in Bowman's capsule and the proximal tubule (Figure 15).
| Figure 15 |
Pressure between blood inside capillary vessels (glomerulus) and urine outside capillary vessels in the Bowman's capsule and the proximal tubule (Figure 16) must be equal (Laws of Physics).
|
| Figure 16 |
Only if equality (pressure equality) exists can free flow across capillaries be ensured. (see Capillary)
Capillaries separate all chemistry elements at equal pressure or hold back because of differences in pressure.
Different pressure between blood inside capillary vessels (glomerulus) and urine outside capillary vessels in the Bowman's capsule and the proximal tubule (Figure 17).
| Figure 17 | 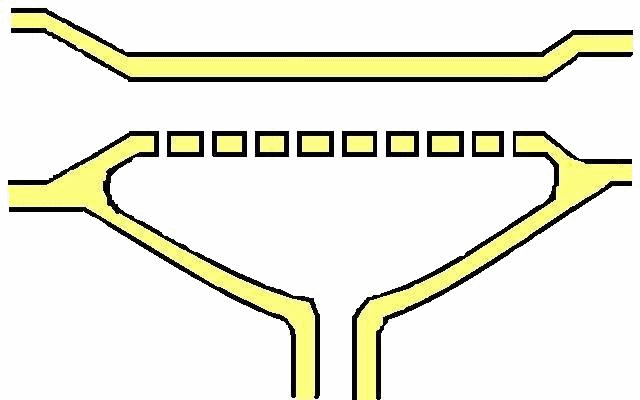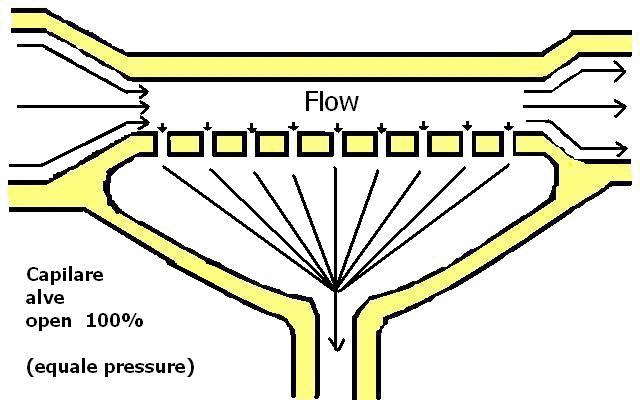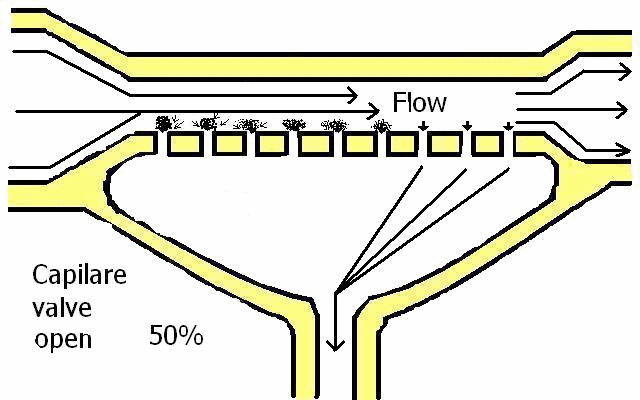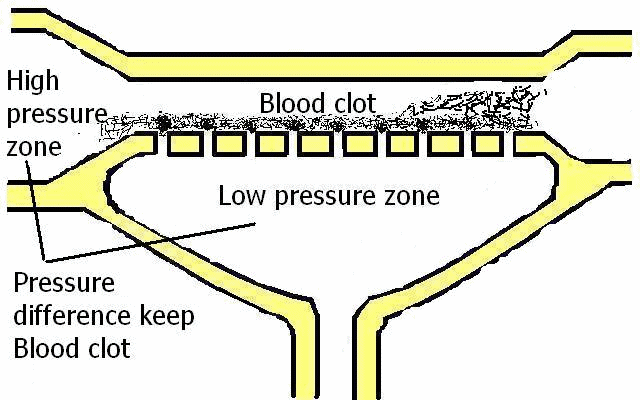 |
Blood pressure varies depending on the body's activities (sleeping, drinking alcohol or coffee, walking, working .....). These activities can move capillary filtration from and back to the Bowman's capsule.
Blood pressure increases and decreases, Blood speed increases and decreases and capillary pressure oscillates and regulates flow. Changes in speed flow change blood consistence in the capillaries (Law of Physics).
Regulation of pressure in the Bowman's capsule is carried out by each organism itself.
GLOMERURAL CLEANING PRINCIPLE
Blood pressure oscillation is the difference in the blood amount; in an analogy with physics, this is only fluid weight expressed as force. This force holds capillary valves open or closed (see next videos for the analogy between blood clots and capillary valves).
More fluid means more force, and more force means more closed capillary valves (Figure 18).
Example:
If pressure A is greater, the capillary valve is closed.
If pressure B is greater, the capillary valve is opened and flow is opposite.
If pressures A and B are equal, the position of the capillary valve is irrelevant (no pressure influence over capillary valve). This means that the capillary valve is leaky in both directions.
| Figure 18 |
Opening Capillary Valve with tweezer (Figure 19)
Figure 19 (see VIDEO)
This law of physics can be used in glomerulus cleaning, by using equal fluid pressure (between blood and urine).
This principle is also used in peritoneal dialysis (see Peritoneal dialysis)..
Dialysis fluid has fluid weight as force and this force opens the capillaries in the stomach (osmotic flow).
(Example: Peritoneal dialysis 1.36%W/V/13.6 mg/m has the chemical formula C6H12O6. This means 6 C atoms and 6 water molecules. 4C (carbon) atoms can include only two ammonia molecules (see Figure 10), or one ammonia molecule and one water molecule (see Figure 11).
These chemistry elements can be included only if pressure is equal.
If the pressure is not equal, capillaries accumulate clots on the high pressure side.
When (blood and urine) pressure in the Bowman's capsule is equal, blood clotting is irrelevant because flow is osmotic and free in both directions.
(See next video demonstration for this law of physics.)
- Fluid pressure is different when fluid height is different, (fluid weight and fluid height are forces).
- Fluid pressure is equal when fluid height is equal.
- Equal fluid height opens capillary osmotic flow (fluid mix in experiment.- See Figure 20).
Figure 20 (see VIDEO)
PRESSURE REGULATING VALVE IMPLANTATION
The pressure regulating valve secures urine flow. The regulating valve is normally closed and it opens with positive pressure (example 18 mmHg). Regulating valve implants are placed in the side of one or both ureters . After implantation, ureters are closed and accumulate urine. When urine pressure comes (for example at 18 mmHg), the regulating valve loses the surplus of urine and by closing, secures a constant (18 mmHg) pressure. This pressure is EQUAL throughout (ureter, renal pelvis, distal tubule, proximal tubule, Bowman's capsule, afferent and efferent arteriole) the whole urinary system.
Equal pressure ensures secretion of urine (see Figure 21).
| Figure 21 |
The pressure regulating valve defines the volume of (ureter and renal pelvis). This volume defines the urine amount and weight (valve opening force).
see Uretric Diameter
Example:
If the urethra and pelvis volume are 2350 mm³ then the approximate max systolic pressure is 168 mmHg and the calculated value for the valve is 168 / 10 = 16.8 = 17 mmHg.
The regulating valve is a micro silicone tube with a diameter of 1.5-4.5 mm and length of 10-25 mm (Figure 22).
Medicine silicone (ELASTOSIL LR liquid silicone) has the most useful properties for valve production.
Figure 22 (see VIDEO)
The pressure regulating valve can have different opening values, depending on the urethra and pelvis volume, or can have one general value (Example: 25 mmHg).
(see Production propose)
CONCLUSION:
Blood disease is now better explained and this explanation insists scientific control.
The kidneys are organs without influence (no scientific chemistry evidence for connection between urea-creatinin and kidney).
Kidneys do not produce chemical elements; Kidneys only accumulate excluded chemistry elements.
That means the exploration focus must be moved from the kidneys to the blood.
It also means that kidney transplantation is not necessary because the disease can be cured by using more effective methods.
Accumulating water in the body is the only result of change in breathing function.
The disease begins if oxygen is missing from the blood, which leads to an increase in CO2 and CH4 gas in blood.
This gas is important because it can produce water as an alternative for body cell breathing.
(See Thesis 2)
(see C4 Carbon Nano Filtration)
Body cells can change from breathing oxygen from air to oxygen from water, and the change causes CH4O2 (hydrocarbon dioxide)
to appear in the blood (Thesis 2).
Water in the lungs and feet is only an accumulating tank for oxygen from water, and the body accumulates water as, for example, fat.
The water accumulation process can be stopped and body cells can use oxygen from the air again.
Author
This investigation began in 2005, prompted by distrust in existing treatment.
At the beginning of part one it was only a hypothesis, but now the hypothesis has been proved.
I learned these laws of physics in my studies more than thirty years ago.
Suddenly, one day ten years ago, I realised the connection.
Now that the work is finished, all that remains to be achieved is practical scientific acknowledgment and utilization.
Vladimir Lazic © March 2011 Denmark
-------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Part three
SINGULARITY LAW
UREA-CREATININ AND HAEMOGLOBIN MOLECULES
Singularity law is dominant in creation Urea-Creatinin and Haemoglobin molecules.
Singularity law is permanent increasing number of chemistry elements.
Increasing numbers example:(see Cell division and Cell cycle).
(see Cell division)
(see Cell cycle)
Singularity example:
Binary division principle ( 1x2=2, 2x2=4, 4x2=8, 8x2=16...∞...):
1 2 4 8 16 32 64 128 256 512 1024 2048 4096 8192 16384 32768 65536 131072 262144...∞...
Singularity law in Urea molecule: (see Figure 23)
| Singularity | one Urea molecule |
| 1 | 1 central atom - Carbon (C) |
| 1 | 1 first shell atoms - Oxigen (O) (separate sig.) |
| 2 | 2 first shell atoms - Nitrogen (N) |
| 4 | 4 second shell atoms - Hydrogen (H) |
| Figure 23 |
Singularity law continues in Creatinin molecule: (see Figure 24)
| Singularity | Creatinin molecule |
| 4 | 4 central atoms - Carbon (C) in 1 Creatinin molecule |
| Figure 24 | 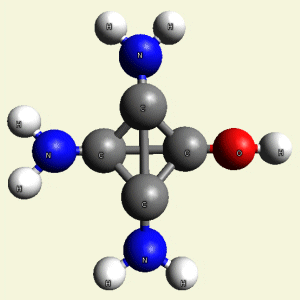
|
Singularity of Creatinin molecules ceases here.
In a Creatinin molecule is missing one Nitrogen and two Hydrogen atoms.
Instead of NH2, an OH (one Oxygen and one Hydrogen atom) is located in Creatinin.
One Oxygen and one Hydrogen atom in a Creatinin molecule. (see Figure 24)
| Nonsingularity Creatinin firste shill (Nitrogen and Oxigen atoms): | |
| Singularity | Creatinin molecule has 3 Nitrogen atoms |
| 1 | 1 Oxygen atom - in 1 Creatinin molecule |
| 3 | 3 Nitrogen atoms - in 1 Creatinin molecule |
| Nonsingularity Creatinin second shill (Hydrogen atoms): | |
| Singularity | Creatinin molecule has 7 Hydrogen atoms |
| 7 | 7 Hydrogen atoms in 1 Creatinin molecule |
Hydrogen and Oxygen can produce electric energy through a mutual reaction.
That electrical energy is enough for an electron shill. Hydrogen and Oxygen are + charged.
This Creatinin molecule with Nitrogen and Hydrogen atoms is - charged.
Four Carbon atoms in the centre are conductive and electric energy is transmitted by electron shill.
Electric potential is a function of electron connection with other electrons.
Singularity law is Evolution law in direction of creating Singular Creatinin molecule
(See CREATING SINGULARY CREATININ MOLECULE)
Singular Creatinin molecule: (see Figure 24a)
Singularity continues in Singular Creatinin first shill:
(1 Creatnin molecule = 4 Nitrogen atoms)
| Singular (S) Creatinin molecule: | |
| Singularity | Creatinin molecules has 4 Nitrogen atoms |
| 4 | 4 Nitrogen atoms - in 1 Creatinin molecule |
Singularity continues in Singular (S) Creatinin second shill:
(1 Creatinin molecule = 8 Hydrogen atoms)
| Singular (S) Creatinin molecule: | |
| Singularity | Creatinin molecule has 8 Hydrogen atoms |
| 8 | 8 Hydrogen atoms in 1 Creatinin molecule |
| Figure 24a |
Singularity continues in increasing number of molecules
| Singularity continues in 4,8,16...Creatinin molecules: | |
| Singularity | Creatinin molecules |
| 16 | 16 central atoms - in 4 Creatinin molecules |
| 32 | 32 central atoms - in 8 Creatinin molecules |
| 64 | 64 central atoms - in 16 Creatinin molecules |
Singularity continues in first shell
| Singularity continues in first shell: | |
| Singularity | Haemoglobin molecule |
| 64 | 64 Nitrogen (N) atoms in 16 Haemoglobin molecules |
| 128 | 128 Nitrogen (N) atoms in 32 Haemoglobin molecules |
| 256 | 256 Nitrogen (N) atoms in 64 Haemoglobin molecules |
Hydrogen atoms theoretic exist in the second shell (see next table)
An example is the NH2 molecule, with one Nitrogen atom and two Hydrogen atoms.
Singularity calculating is:
number Nitrogen atoms x 2 = number Hydrogen atoms.
| Singularity continues in second shell: | |
| Singularity | Haemoglobin molecules |
| 512 | 512 Hydrogen (H) atoms in 64 Haemoglobin molecules |
| 1024 | 1024 Hydrogen (H) atoms in 128 Haemoglobin molecules |
|
| Singularity continues in Haemoglobin cells: | |
| Singularity | Haemoglobin cells |
| 1024 | 1024 Haemoglobin molecules in 256 Haemoglobin cells |
| 2048 | 2048 Haemoglobin molecules in 512 Haemoglobin cells |
| 4096 | 4096 Haemoglobin molecules in 1024 Haemoglobin cells |
| Singularity continues in Haemoglobin cells ( first shill) | |
| Singularity | Haemoglobin cells |
| 4096 | 4096 Nytrogen (N) atoms in 256 Haemoglobin cells |
| 8192 | 8192 Nytrogen (N) atoms in 512 Haemoglobin cells |
| 16384 | 16384 Nytrogen (N) atoms in 1024 Haemoglobin cells |
CREATING A SINGULAR (S) CREATININ MOLECULE:
The solidification process in one Creatinin molecule excludes NH3 (ammonia) molecules See Solidification process
With the NH3 ammonia molecule, a second Creatinin molecule changes. See Ammonia data
The Creatinin molecule includes 1 Nytrogen atom and 2 Hydrogen atoms NH2 and excludes Oxygen and 2 Hydrogen atoms and the result is: H2O water molecule.
| C4H7N3O + NH3→ C4H8N4 excludes H2O |
Creatinin molecule include 1 Nytrogen atom and 2 Hydrogen atoms and exclude Oxigen and 2 Hydrogen atoms and become
Singular Creatinin molecule C4H8N4
( see Figure 25)
| Figure 25 | 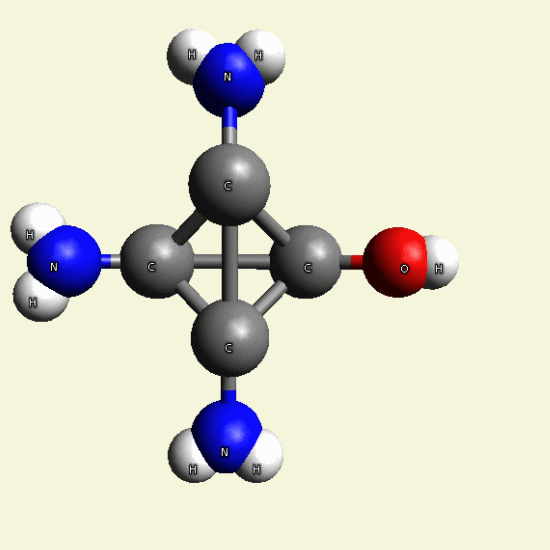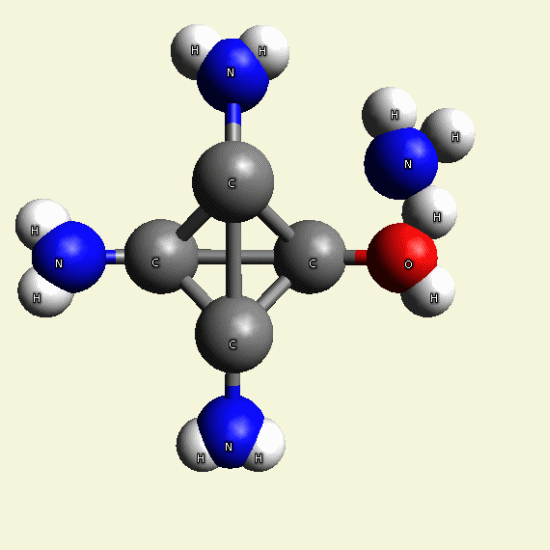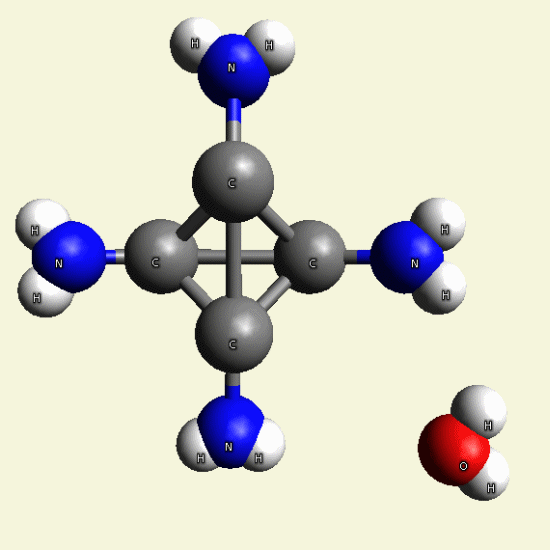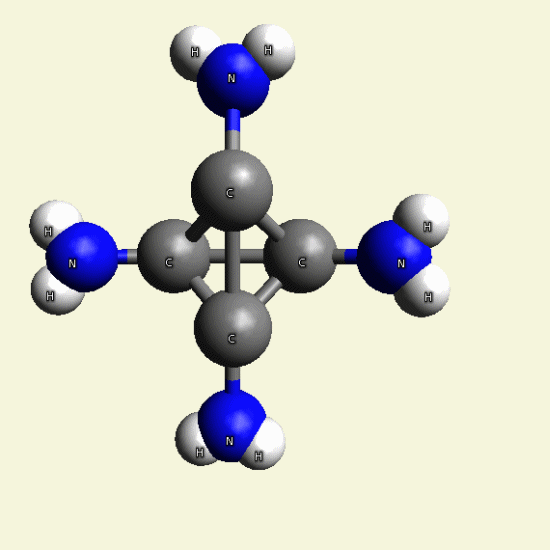
|
|
|
This S Creatinin molecule evolves in accordance of the Singularity low.
With increasing combination of chemistry elements, this S Creatinin molecule exists.
The lifetime of these molecules is short because the chemical process continues with solidification.
Singularity low can be used for calculating:
Urea-Creatinin-Haemoglobin value.
Oxigen and Water the body needs.
Determining the Urea-Creatinine value.
VITAMINS
We must know that Urea -Creatinin can change condition from Urea in Creatinin and from Creatinin in Urea.
This change is the result of missing B vitamins:
Vitamins B6 and B12 must be in balance for a stable Urea-Creatinin value.
If vitamin B6 is missing, the Urea value increases.
B6 Vitamin C8H11NO3
See Vitamin B6
See Vitamin B6
See Pyridoxine
If vitamin B12 missing, the Creatinin value increases.
B12 Vitamin C61-64H84-90N14 O13-14PCo
See Vitamin B12
See Vitamin B12
See Vitamin B12 benefits
Vitamin B12 contains a Cobalt ion at the centre of the Porphyrin
See Porphyrin
See Cobalt
Ferophorpherin (see Figure 26)
| Figure 26 |
| Haemoglobin molecule (see Figure 27). |
| Figure 27 |
| Haemoglobin contains an Iron atom at the centre of the molecule: |
| This illustrates the B12 content that is in the connection with vitamin missing. |
| When using vitamins B6, B12 and C it is possible to decrease both Creatinin and Urea values. |
| See Vitamin C 3D |
| See Vitamin -benefits |
| See Vitamins cause |
SOLIDIFICATION PROCESS FOR A SINGULAR CREATININ MOLECULE
The solidification process attracts central C4 carbon atoms to the centre of the moleculeand excludes atoms from the periphery.
Singular Creatinin molecule disintegrates in:
- carbon C4 molecule with a covalent bond between two C atoms. see Carbon data
- four NH2 molecules.
Solidification for Singulary Creatinin molecule. (see Figure 28)
| Figure 28 |  |
CREATION OF TWO UREA MOLECULES (NH2)2CO
The chemical process continues with four NH2 molecules and two CO2 (Carbon dioxide) molecules.
Two NH2 molecules include CO and exclude O Oxigen atom, from CO2 (Carbon dioxide) molecules.
| NH2 + NH2 + CO2 →(NH2)2CO excludes O |
| NH2 + NH2 + CO2 →(NH2)2CO excludes O |
The excluded Oxygen atom has chem. Process O + O → O2
Creation of two Urea molecules. (see Figure 29)
| Figure 29 |  |
TRANSFORMATION OF CREATININ MOLECULE |
(See Hypothesis 2) |
HAEMOGLOBIN MOLECULE (FeN4 CORE)
The chemical process can change with the chemical synthesis of Fe (Iron Atom), and four NH2 molecules from solidification of the S Creatinin molecule (see Solidification S Creatinin mol.) and CO2 (Carbon dioxide) molecules.
The four NH2 molecules include Fe and exclude H8
The Oxigen atom, from CO2 (Carbon dioxide) molecules, can form 2H2O and CH4.
The chemical process is::
(NH2)4 + Fe + CO2 → FeN4(Haemoglobin molecule core) excludes CH4 and excludes 2H2O
See Ferophorpherin
See Hemoglobin.
Excluded CH4 and 2H2O continue the chemical process in direction of creating another S Creatinin molecule.
The chemical process is:
CH4 + excluded O2 → CH4O2
See Methane
2H2O → H4O2 → H4 + (O2 + C ) → CH4O2
The chemical process for CH4 is:
CH4 + O2 → 2H2O excludes C
C + O2 → CO2
or
CH4 + O2 → CO2 excludes H4
H4 + O2 → 2H2O
Figure 30

COMBINATION OF SINGULARITY LAW (ANOTHER DISEASE)
| Figure 30 | 
|
Basic chemical elements for breathing are:
- O2 Oxigen molecule
- C Carbon atom
- CO2 Carbon dioxide
- H2O Water molecule...Cell contents are more and 90% water
In the breathing process the cell absorbs an O2 Oxygen molecule and extracts CO2 Carbon dioxide.
The cell in breading process absorb O Oxigen molecule, and extract CO2 Carbondioxide, H2O content is unchanged.
This is healthy breathing process.
Failure of the cell breathing process.
With failure of the cell breathing process,
the cell absorbs an CH4 Methane molecule, but extracts a O2 Oxygen molecule.
This methane molecule is the product of a chemical process:
C + 2H2O → CH4 extractes O2 Oxigen atoms.
This O2 Oxigen molecule, connects with the C Carbon atom and becomes CO2 Carbon dioxide.
New chem. reaction.:
CO2 + CH4→ C2 extracte 2H2O
and vice versa:
CO2 + CH4 → 2H2O extracte C2
In the first case, the cell increases the Singular number of C Carbon atoms.(see bio amino acid)
In the second case, the cell increases the Singular number of 2H2O Water molecules.
The cell content (C Carbon) divides into two atoms (1x2=2, 2x2=4, 4x2=8, 8x2=16...?) and C Carbon atoms content in every cell increases in accordance with the singularity low.
This cell is maybe the first cancer cell.
The cell with 2H2O atoms content is maybe the first leukaemia cell.
Leukaemia can be a product of a normal evolutional process, out of control.
See normal process excluding H2O and 2H2O:
see UREA
see UREA - CREATININ DISINTEGRATION
see SOLIDIFICATION PROCESS FOR CREATININE
This process is acknowledgment for Hypothesis 4 see Hypothesis 4
Binary singularity
The excluded O Oxygen atom, from the CO2 (Carbon dioxide) molecule continues the chemical process, with one of singularity mode:
see Creation of two Urea molecules
see Creation of Urea
see UREA
The three chemical processes are the same all the time; only the number of singularities changes.(see Figure 31)
LIFETIME AND CHEMICAL PROCESS
The number 1-2-4 is the lifetime of a Urea molecule.It is seven singular periods long..
That lifetime is the time in that form. Molecules transform all the time and in that period has UREA form.
The number 8-16-32 is the lifetime for Creatinin molecules. It is 56 singular periods long.
The number 64 is 64 singular periods long, and that is the lifetime for S Creatinin molecule.
The number 128 is 128 singular periods long, and that is the lifetime for Haemoglobins FeN4 core. (see Hemoglobin).
These numbers (from 1 to 128) were beginning of the singularity in the later period when the singularity had the greatest number of molecules and combinations, this lifetime is the same.
Increases only number of all molecules.(see calculator)
Figure 31
| Sin. | Chemical process |
| CO2 + 2H2O → CO2 + H4O2→ CH4 + O2 → CH4O2 + N2 → (NH2)2CO [first Urea] excludes O excludes O2 | |
| excluded O + 2H2O → H4 + (O2 + C) + O → CH4 + O3 → CH4 + O + N2 → (NH2)2CO [second Urea] excludes O2 | |
| excluded O2 + C → CO2 + N2H4 → (NH2)2CO [thrd Urea] excludes O excluded O2 + C → CO2 + N2H4 → (NH2)2CO [fourt Urea] excludes O excluded O + O → O2 excludes O2 |
|
| (NH2)2CO + (NH2)2CO + (NH2)2CO + (NH2)2CO → C4H16N8O4 → C4H7N3O excludes H9N5O3 + excluded O2 | |
| excluded O2 + C → CO2 + excluded H9N5O3 →
CH9N5O3 excludes O2 excluded O2 + C → CO2 + CH9N5O3 → C2H9N5O3 excludes O2 excluded O2 + C → CO2 + C2H9N5O3 → C3H9N5O3 excludes O2 excluded O2 + C → CO2 + C3H9N5O3 → C4H9N5O3 → C4H7N3O excludes H2N2 excludes 2O2 New Urea branch initiaion is excluded H2N2 + excluded 2O2 → H2N2 + H2O + CO2 + O →(NH2)2CO excludes O excludes O2 |
|
| C4H7N3O Solidification excludes C4 + NH3 + H2O + H2N2 and share in: NH3 + C4H7N3O → C4H8N4 excludes H2O H2O + H2N2 + CO2 →(NH2)2CO excludes O2 excluded H2O + excluded O2 → H2O + CO2 + H2N2 → (NH2)2CO excludes O2 |
|
| C4H8N4 Solidification excludes C4 excludes (NH2)4 (NH2)4 + CO2 + CO2 → (NH2)2CO + (NH2)2CO |
|
| (NH2)2 + (NH2)2 + Fe + CO2 → FeN4 excludes CH4 excludes H2O + H2O |
The next table calculator can explain , the different evolution phases.(see calculator)
For calculating of evolution phases we can use next calculator for different molecules.
Example:
Write the Urea value 20 mmol/l in first cell, and see haw meny molecules have certain phase.
| mmol/l. | Sin. | Chemical process | |
| 1 | (NH2)2CO first Urea molecules | ||
| 2 | (NH2)2CO second Urea molecules | ||
| 4 | (NH2)2CO fourt Urea molecules | ∞ | Number of molecules |
Example:
Creatinin value is 1000 mmol/l, this value can be calculated because we know the phase.
We know that blood content is 1000 mmol/l of Creatinin, and we know how many molecules have Creatinin form.
The Creatinin content value is important, and the evolution phase is life cycle for the Creatinin molecule.
Creatinin value is average number of molecules in the while, and that number increases.. (see Law of Singularity)
| mmol/l. | Sin. | Chemical process | |
| 8 | C4H7N3O first Creatinin molecules | ||
| 16 | C4H7N3O Creatinin molecules (O cicle) | ||
| 32 | C4H7N3O Creatinin molecules (Solidification) | ∞ | Number of molecules |
NEW SINGULARITY BRANCH
Singularity 32 is specific because it contains three chemical processes (see Figure 31 singularity 32).
The content for that singularity is :
C4H7N3O Creatinin molecules
C4H8N4 S Creatinin molecules
(NH2)2CO first phase Urea molecules
New Singularity bigins with 1 Creatinin molecule that excludes:
one NH3 ammonia molecule
two NH3 ammonia molecules
in the Creatinin solidification process.(See Solidification process)
That ammonia connects with another one or two Creatinin molecules and become one or two Singular Creatinin molecules. The one or two Singular Creatinin molecules in the solidification process, exclude 4 or 8 NH2 molecules.(See S Solidification process)
That creates 2 or 4 (NH2)2CO Urea molecules (See Creation two Urea molecules).
Singularity is (1 1 2) and (1 2 4) (Creatinin, S Creatinin, Urea).
The next table calculator can explain Singularity 32.
The previous example for singularity 32 was 571.4285714285714 Creatinin molecules.
In calculating we use (see 1 2 4 Sin. calculator)
The result is in the next table.
| mmol/l. | Sin. | Chemical process | |
| 1 | C4H7N3O Creatiine molecules | ||
| 2 | C4H8N4 Singulary Creatinine molecules | ||
| 4 | (NH2)2CO Urea molecules | ∞ | Number of molecules |
Singularity 64 is simple, 1 S Creatinin molecule becomes 2 two-phase Urea molecules.
The result of multiplying number is 163.14285714285714 by 2.
163.14285714285714 * 2 = 326 two phases (NH2)2CO Urea molecules. (see front table sin 4)
The 326 two phase (NH2)2CO Urea molecules, cannot be created,
if Fe (Iron) content in blood is over the maximum value.(see Singularity 128)
Singularity 128
In Singularity 128 the chemical process can be stopped, if the blood content has more than the maximum number of Fe Iron atoms.
The chemical process has two directions:
see Creation of Haemoglobin molecule
Chemical process Fe + N4 → FeN4 one Haemoglobin molecule core.
Chemical process 2H2O → H4 + (O2+ C) → CH4O2 but N2 is missing to create an Urea molecule
Chemical process CH4 + O2 → CH4O2 but N2 is missing to create Urea molecule.
That 2N2 is connected in the FeN4 Haemoglobin molecule core, and creation of Urea molecule can be stoped.
CONCLUSION
A singularity is a chemical evolution, and that evolution is normal for all people.
Chemistry elements are missing and chemical process is changing.
This change is an unfavourable chemical process.
The blood must all time contents more than maximum Fe (Iron atoms), and O (Oxygen).
Food must contain Iron. Oxygen can be consumed with physical activity.
Singularity law is a possibility for distribution of evolution phases and the number of molecules and content of different atoms in molecules.(see calculator)
Author
This work has a new point of view for Kidney disease and it is the product of more years of theoretical work.
It is only the beginning of serious scientific exploration of this subject.
Vladimir Lazic Denmark September 2011
------------------------------------------------------------------------------------------------------------------
Part four
ANALYTICAL CHEMISTRY OF THE CREATININ MOLECULE
See Analytical Chemistry
The chemical process can change the content of creatinin molecules, with the chemical synthesis of C4 atoms.
The Carbon atom connection with another carbon atom, determines the kind of molecules. See Carbon bond
C to C4 Evolution is the lifetime of the creatinin molecule; the last step is solidification and the beginning of the next singularity.
| Evolution of carbon atoms core in creatinin molecule | ||||
| C | C2 | C3 | 2C2 | C4 |
See Singular and Binary table
See Oxocarbon
ANALYTICAL PROCESS FOR A NONSINGULAR CREATININ MOLECULE C4H7N3O
Example 1 |
Singularity one
One Creatinin molecule C4H7N3O disintegrates in: See right side C form- C atom See Carbon
- CH4 molecule See Methane
- NH3 molecule See Ammonia
- CO molecule See Carbon monoxide
- CN2 molecule (See Figure 31A)
| C excludes CH4 excludes NH3 excludes CO excludes N2 |
Creatinin molecule content with C atom in Figure 31A
| Figure 31A | Molecule content | ||||
 |
 |
||||
| C | CH4 | NH3 | CO | CN2 | |
Chemical multiple synthesis : See Chemical synthesis
| C + CH4 + NH3 + CO + CN2 → C4H7N3O |
C Creatinin molecule in Figure 31B
| Figure 31B |
Example 1A |
Singularity two
One Creatinin molecule disintegrates in: See right side C2 form- C2 molecule with a four covalence bond between atoms. See Carbon
- CH4 molecule See Methane
- NH3 molecule See Ammonia
- CO molecole See Carbon monoxide
- N2 molecule See Nitrogen
| C2 excludes CH4 excludes NH3 excludes CN2O |
Creatinin molecule content with C2 atoms in (Figure 32)
| Figure 32 | Molecule content | ||||
 |
 |
||||
| C2 | CH4 | NH3 | CO | N2 | |
| See C2 Creatinin molecule |
|---|
Chemical multiple synthesis : See Chemical synthesis
| C2 + CH4 + NH3 + CO + N2 → C4H7N3O |
C2 Creatinin molecule in (Figure 33)
| Figure 33 | 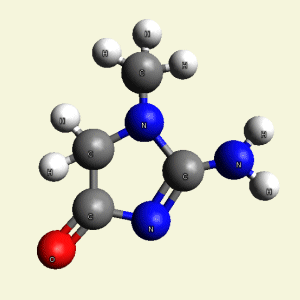 |
Example 2 |
Singularity three
One Creatinin molecule disintegrates in: See right side C3 form- C3 molecules with a two-covalence bond between atoms See Carbon
- CH4 molecule See Methane
- H2O molecule See Water
- HN3 molecule (See Figure 34)
| C3 excludes CH4 excludes H2O excludes HN3 |
Creatinin molecule content with C3 atoms in (Figure 34)
| Figure 34 | Molecule content | |||
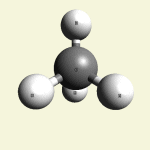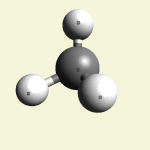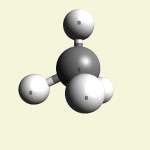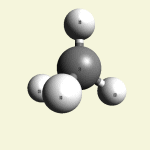 |
 |
 |
||
| C3 | CH4 | H2O | HN3 | |
Chemical multiple synthesis : See Chemical synthesis
| C3 + CH4 + H2O + HN3 → C4H7N3O |
C3 Creatinin molecule in (Figure 35)
| Figure 35 |  |
Example 2A |
One Creatinin molecule disintegrates in:
- C3 molecules with a two-covalence bond between atoms. See Carbon
- CH4 molecule See Methane
- NH3 molecule See Ammonia
- N2O molecule See Nitrogen
| C3 excludes CH4 excludes NH3 excludes N2O |
Creatinin molecule content with C3 atoms in (Figure 36)
| Figure 36 | Molecule content | |||
 |
 |
 |
||
| C3 | CH4 | NH3 | N2O | |
Chemical multiple synthesis : See Chemical synthesis
| C3 + CH4 + NH3 + N2O → C4H7N3O |
C3 Creatinin molecule in (Figure 37)
| Figure 37 |  |
Example 3 |
Singularity four
One Creatinin molecule disintegrates in: See right side C4 form- 2C2 molecules with a two-covalence bond between atoms. See Carbon
- NH3 molecule See Ammonia
- H2O molecule See Water
- H2N2 molecule (see Figure 38)
| 2C2 excludes NH3 excludes H2O excludes H2N2 |
Creatinin molecule content with 2C2 atoms in (Figure 38)
| Figure 38 | Molecule content | |||
 |
 |
 |
||
| 2C2 | NH3 | H2O | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| 2C2 + NH3 + H2O + H2N2 → C4H7N3O |
2C2 Creatinin molecule in (Figure 39)
| Figure 39 | 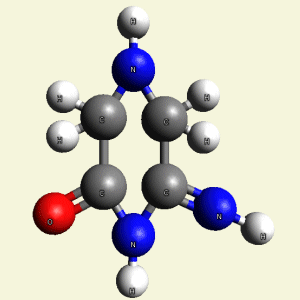 |
Example 4 |
See SOLIDIFICATION PROCESS FOR CREATININE
One Creatinin molecule disintegrates in: (See Figure 11)
- C4 molecule with a covalent bond between atoms. See Carbon
- NH3 molecule See Ammonia
- H2O molecule See Water
- H2N2 molecule (See Figure 40)
| C4 excludes NH3 excludes H2O excludes H2N2 |
Creatinin molecule content in (Figure 11 and 40)
| Figure 40 | Molecule content | |||
 |
 |
 |
||
| C4 | NH3 | H2O | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C4 + NH3 + H2O + H2N2 → C4H7N3O |
C4 Creatinin molecule in (Figure 41)
| Figure 41 | 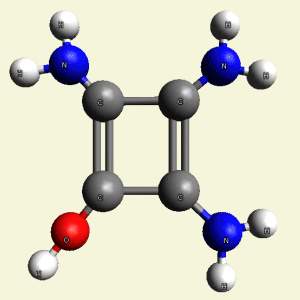 |
Example 4A |
See SOLIDIFICATION PROCESS FOR CREATININ
One Creatinin molecule disintegrates in: (See Figure 11)
- C4 molecule with a covalent bond between atoms. See Carbon
- NH3 molecule See Ammonia
- H2O molecule See Water
- H2N2 molecule (See Figure 40A)
| C4 excludes NH3 excludes H2O excludes H2N2 |
Creatinin molecule content in (Figure 11 and 40A)
| Figure 40A | Molecules content | |||
 |
 |
 |
||
| C4 | NH3 | H2O | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C4 + NH3 + H2O + H2N2 → C4H7N3O |
C4 Creatinin molecule in (Figure 41A)
| Figure 41A | 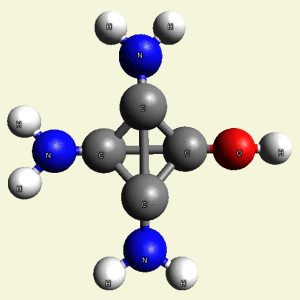 |
Example 4B |
Two Creatinin molecules:(See Figure 10)
| Two Creatinin molecules disintegrate in: - 2C4 molecule with a covalent bond between atoms. See Carbon - 4NH3 molecule See Ammonia - H2O2 molecule See Hydrogen peroxide - N2 molecule See Nitrogen |
ANALYTICAL PROCESS FOR SINGULAR CREATININ MOLECULE C4H8N4
Example 5 |
Singularity one
One Creatinin molecule C4H8N4 disintegrates in:
- C atom See Carbon
- 2CH4 molecules See Methane
- CN4 molecule See Figure 41B
| C excludes 2CH4 excludes CN4 |
C Creatinin molecule content in Figure 41B
| Figure 41B | Molecule content | ||||
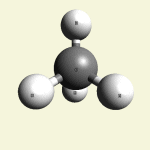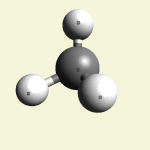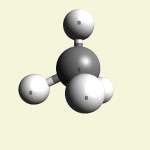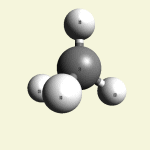 |
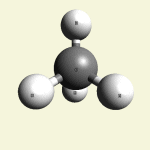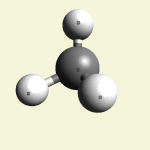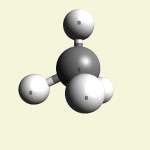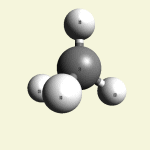 |
||||
| C | CH4 | CH4 | CN4 | ||
Chemical multiple synthesis : See Chemical synthesis
| C + 2CH4 + CN4 → C4H8N4 |
C Creatinin molecule in Figure 41C
| Figure 41C |
Example 5A |
Singularity two
One Creatinin molecule disintegrates in:
- C2 molecule with a two-covalence bond between atoms. See Carbon
- 2CH4 molecules See Methane
- N4 molecule (See Figure 42)
| C2 excludes 2CH4 excludes N4 |
Creatinin molecule content with C2 atoms in (Figure 42)
| Figure 42 | Molecule content | |||
 |
 |
 |
||
| C2 | CH4 | CH4 | N4 | |
Chemical multiple synthesis: See Chemical synthesis
| C2 + 2CH4 + N4 → C4H8N4 |
C2 Creatinin molecule in (Figure 43)
| Figure 43 | 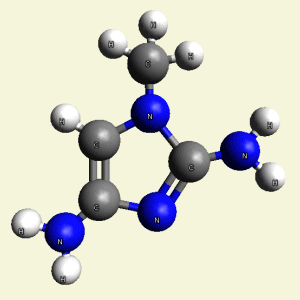 |
Example 6 |
Singularity three
One Creatinin molecule disintegrates in:
- C3 molecule with a covalence bond between atoms. See Carbon
- CH4 molecule See Methane
- 2H2N2 molecule (See Figure 44)
| C3 excludes CH4 excludes 2H2N2 |
Creatinin molecule content with C3 atoms in (Figure 44)
| Figure 44 | Molecule content | |||
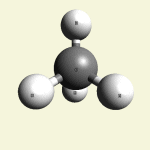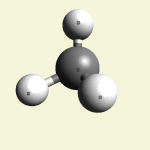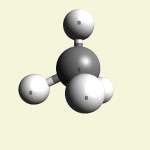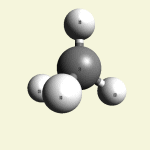 |
 |
|||
| C3 | CH4 | 2H2N2 | ||
Chemical multiple synthesis: See Chemical synthesis
| C3 + CH4 + 2H2N2 → C4H8N4 |
C3 Creatinin molecule in (Figure 45)
| Figure 45 |  |
Example 6A |
One Creatinin molecule disintegrates in:
- C3 molecule with a covalence bond between atoms. See Carbon
- CH4 molecule See Methane
- NH3 molecule See Ammonia
- HN3 molecule (See Figure 46)
| C3 excludes CH4 excludes NH3 excludes HN3 |
Creatinin molecule content with C2 atoms in (Figure 46)
| Figure 46 | Molecule content | |||
 |
 |
 |
||
| C3 | CH4 | NH3 | HN3 | |
Chemical multiple synthesis: See Chemical synthesis
| C3 + CH4 + NH3 + HN3 → C4H8N4 |
C3 Creatinin molecule in (Figure 47)
| Figure 47 | 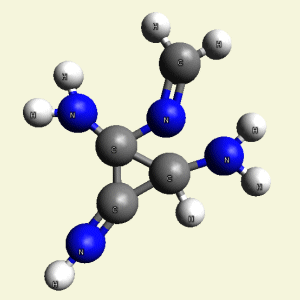 |
Example 6B |
One Creatinin molecule disintegrates in:
- C3 molecule with a covalence bond between atoms. See Carbon
- 2NH3 molecules See Ammonia
- CH2N2 molecule (See Figure 48)
| C3 excludes 2NH3 excludes CH2N2 |
Creatinin molecule content with C3 atoms in (Figure 48)
| Figure 48 | Molecule content | |||
 |
 |
 |
||
| C3 | NH3 | NH3 | CH2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C3 + 2NH3 + CH2N2 → C4H8N4 |
C3 Creatinin molecule in (Figure 49)
| Figure 49 | 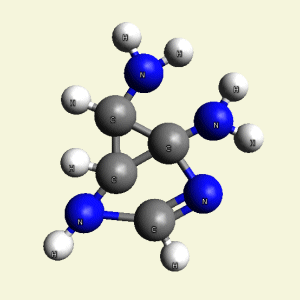 |
Example 7 |
Singularity four
One Creatinin molecule disintegrates in:
- 2C2 molecules with a covalence bond between atoms. See Carbon
- 4NH2 molecules (See Figure 50)
| 2C2 excludes NH2 excludes NH2 excludes NH2 excludes NH2 |
See S Solidification process
See Creation of two Urea molecules
Creatinin molecule content with 2C2 atoms in (Figure 50)
| Figure 50 | Molecule content | ||||
 |
 |
 |
 |
||
| 2C2 | NH2 | NH2 | NH2 | NH2 | |
Chemical synthesis: See Chemical synthesis
| 2C2 + 4NH2 → C4H8N4 |
2C2 Creatinin molecule in (Figure 51)
| Figure 51 | 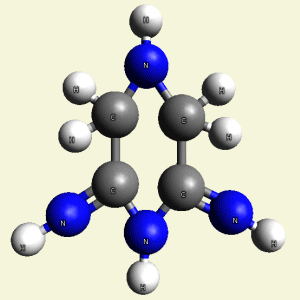 |
Example 7A |
One Creatinin molecule disintegrates in:
- 2C2 molecule with a covalence bond between atoms. See Carbon
- 2NH3 molecules See Ammonia
- H2N2 molecule (See Figure 52)
| 2C2 excludes 2NH3 excludes H2N2 |
Creatinin molecule content with 2C2 atoms in (Figure 52)
| Figure 52 | Molecule content | |||
 |
 |
 |
||
| 2C2 | NH3 | NH3 | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| 2C2 + 2NH3 + H2N2 → C4H8N4 |
2C2 Creatinin molecule in (Figure 53)
| Figure 53 | 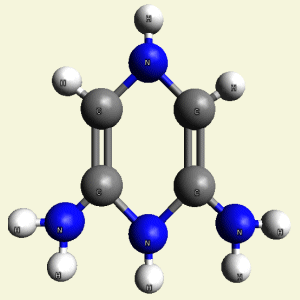 |
Example 8 |
One Creatinin molecule disintegrates in:
- C4 molecule with a covalence bond between atoms. See Carbon
- 4NH2 molecules (See Figure 54)
| C4 excludes NH2 excludes NH2 excludes NH2 excludes NH2 |
Creatinin molecule content with C4 atoms in (Figure 54)
| Figure 54 | Molecule content | |||
 |
 |
 |
 |
|
| C4 | NH2 | NH2 | NH2 | NH2 |
Chemical synthesis: See Chemical synthesis
| C4 + 4NH2 → C4H8N4 |
C4 Creatinin molecule in (Figure 55)
| Figure 55 | 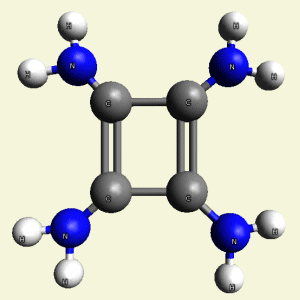 |
See S Solidification process
See Creation of two Urea molecules
Example 8A |
Creatinin molecule disintegrates in:
- C4 molecule with a covalence bond between atoms. See Carbon
- 2NH3 molecules See Ammonia
- H2N2 molecule (See Figure 56)
| C4 excludes 2NH3 excludes H2N2 |
Creatinin molecule content with C4 atoms in (Figure 56)
| Figure 56 | Molecule content | |||
 |
 |
 |
||
| C4 | NH3 | NH3 | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C4 + 2NH3 + H2N2 → C4H8N4 |
C4 Creatinin molecule in (Figure 57)
| Figure 57 | 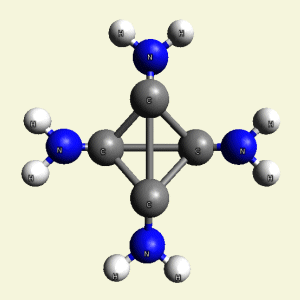 |
CREATING EQUAL (E) CREATININ MOLECULE:
The solidification process in one Creatinin molecule See Figure 31 (singularity 128) was 50% transformation.
Example from (singularity 128 from Figure 31)
| (NH2)2 + (NH2)2 + Fe + CO2 → FeN4 excludes CH4 excludes H2O + H2O |
100% transformation of Creatinin molecule, have to four water molecules (4H2O). See Water
Singularity example for water:
Example A |
- One H2O molecule See CREATING SINGULARY CREATININ MOLECULE
- Two H2O molecules See Figure 31 singularity 128
- Four H2O molecules See chemical process
Example B |
A quark is a result of an empty singularity. See quark
An electron is a result of a quark singularity. See electron
A proton is a result of a quark singularity. See proton
A neutron is a result of a quark singularity. See neutron
A nucleus is a result of a electrons equality. See nucleus
EQUALITY
A hydrogen atom is a result of equality in singularity one.
Equality of (one proton, one neutron, one electron)
See Hydrogen
See Hydrogen
See Hydrogen
An oxygen atom is a result of equality in singularity eight.
Equality of (eight protons, eight neutrons, eight electrons (2 and 6) )
See Oxygen
The chemical process singularity one is:
One hydrogen atom + One oxygen atom → HO
| H + O → HO |
The chemical process singularity two is:
| HO + HO → H2O2 |
Equality of(two hydrogen atoms, two oxygen atoms)
See Hydrogen peroxideThe chemical process Water singularity one is:
| H2O2 → H2O excludes O |
The chemical process Water singularity two is:
| H2O + H2O → 2H2O |
The chemical process Water singularity four is:
| 2H2O + 2H2O → 4H2O |
Creation of Equal (E) Creatinin molecule C4H4O4
(See Figure 58)Equality of (four carbon atoms, four hydrogen, four oxygen atoms)
A singular (S) Creatinin molecule includes 4H2O (four water molecules) and excludes 4NH3 (four ammonia molecules) and becomes an Equal Creatinin molecule C4H4O4 (See Figure 58)
| C4H8N4 + 4H2O → C4H4O4 excludes 4NH3 |
| Figure 58 | 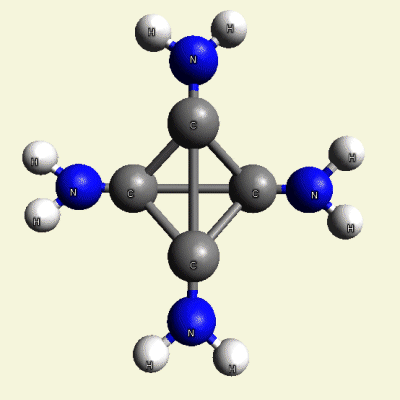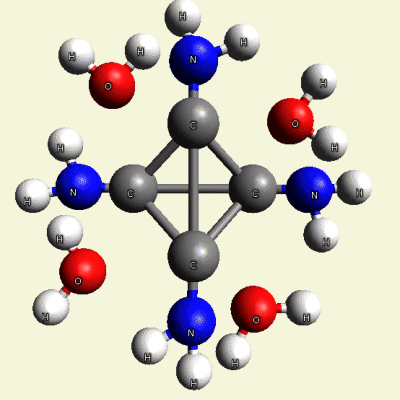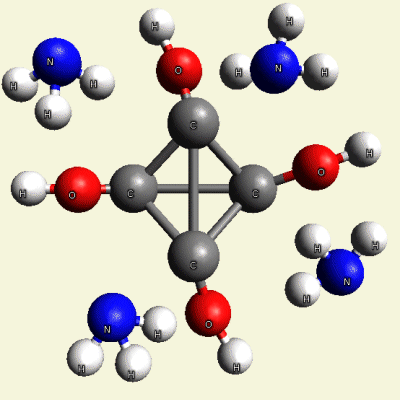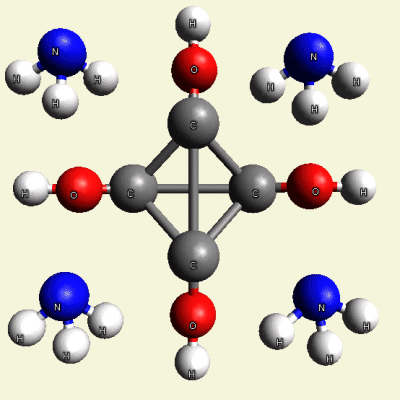 |
C4H4O4 4NH3 |
ANALYTICAL PROCESS FOR EQUAL CREATININ MOLECULE C4H4O4
Equal Creatinin molecule C4H4O4 (See Figure 59)
| Figure 59 |
The chemical process in 50% transformation See chemical process
Example 8B |
Singularity one
One Creatinin molecule C4H4O4 disintegrates in:- C atom See Carbon
- CH4 molecule See Methane
- 2CO2 molecules See Carbon dioxide
| C excludes CH4 excludes 2CO2 |
C Creatinin molecule content in Figure 59A
| Figure 59A | Molecule content | ||||
 |
|||||
| C | CH4 | CO2 | CO2 | ||
Chemical multiple synthesis : See Chemical synthesis
| C + CH4 + 2CO2 → C4H4O4 |
C Creatinin molecule in Figure 59B
| Figure 59B |
Example 9 |
Singularity two
(E) Creatinin molecule disintegrates in:
- C2 molecule with a covalent bond between atoms. See Carbon
- CH4 molecule See Methane
- CO2 molecule See Carbon dioxide
- O2 molecule see Oxygen
| C2 excludes CH4 excludes CO2 excludes O2 |
(E) Creatinin molecule content with C2 atoms in (Figure 60)
| Figure 60 | Molecule content | |||
 |
 |
|||
| C2 | CH4 | CO2 | O2 | |
Chemical multiple synthesis: See Chemical synthesis
| C2 + CH4 + CO2 + O2 → C4H4O4 |
C2 Creatinin molecule in (Figure 61)
| Figure 61 | 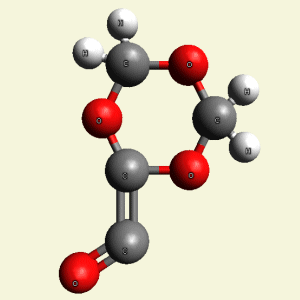 |
Example 10 |
Singularity three
(E) Creatinin molecule disintegrates in:
- C3 molecule with a covalent bond between atoms. See Carbon
- CO2 molecule See Carbon dioxide
- 2H2O molecules See Water
| C3 excludes CO2 excludes 2H2O |
Creatinin molecule content with C3 atoms in (Figure 62)
| Figure 62 | Molecule content | |||
 |
 |
|||
| C3 | CO2 | H2O | H2O | |
Chemical multiple synthesis: See Chemical synthesis
| C3 + CO2 + 2H2O → C4H4O4 |
C3 Creatinin molecule in (Figure 63)
| Figure 63 | 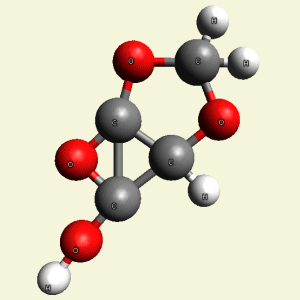 |
Example 11 |
Singularity four
(E) Creatinin molecule disintegrates in:
- 2C2 molecules with a covalent bond between atoms. See Carbon
- 2H2O molecules See Water
- O2 molecule See Oxygen
| 2C2 excludes 2H2O excludes O2 |
Creatinin molecule content with 2C2 atoms in (Figure 64)
| Figure 64 | Molecule content | |||
 |
 |
 |
||
| 2C2 | H2O | H2O | O2 | |
Chemical multiple synthesis: See Chemical synthesis
| 2C2 + 2H2O + O2 → C4H4O4 |
2C2 Creatinin molecule in (Figure 65)
| Figure 65 | 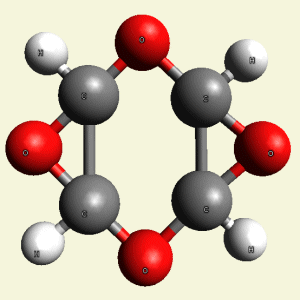 |
Example 12 |
(E) Creatinin molecule disintegrates in:
- C4 molecule with a covalent bond between atoms. See Carbon
- 2H2O molecules See Water
- O2 molecule See Oxygen
| C4 excludes 2H2O excludes O2 |
Creatinin molecule content with C4 atoms in (Figure 66)
| Figure 66 | Molecule content | |||
 |
 |
 |
||
| C4 | H2O | H2O | O2 | |
Chemical multiple synthesis: See Chemical synthesis
| C4 + 2H2O + O2 → C4H4O4 |
C4 Creatinin molecule in (Figure 67)
| Figure 67 |
ANALYTICAL PROCESS FOR EQUAL CREATININ MOLECULE C4H4O4 + 4NH3
Equal Creatinin molecule (without Carbon atoms) + four Ammonia molecule. (See Figure 68)
The chemical process 100% transformation See chemical process
Chemical synthesis: See Chemical synthesis
| HO + HO + HO + HO → 2H2O2 |
| 2H2O2 + 4NH3 |
| Figure 68 | 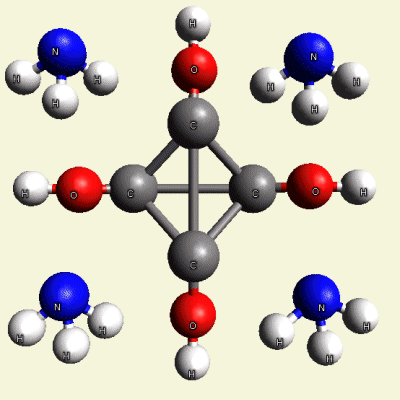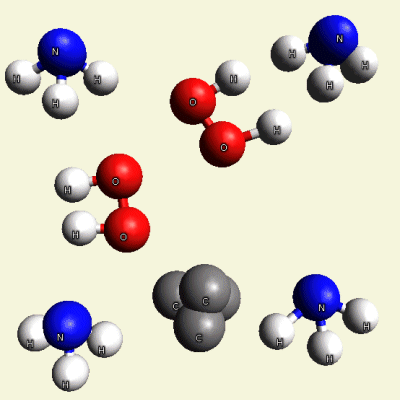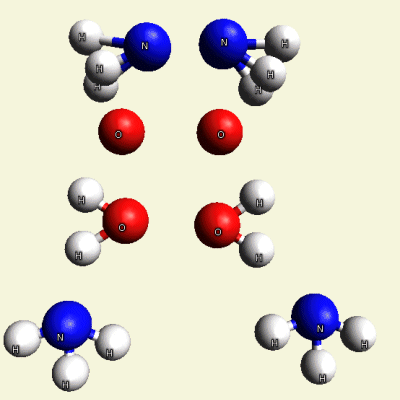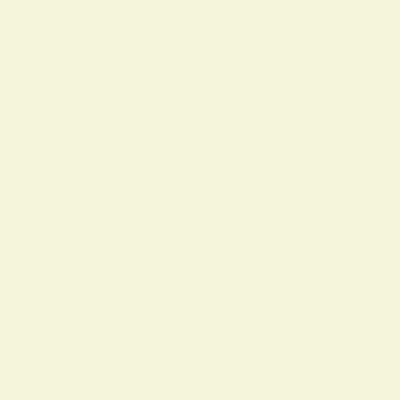 |
Example 13 |
Chemical analysis: See Analytical chemistry
(E) Creatinin molecule disintegrates in:
- 4H2O molecules See Water
- 2NH3 molecules See Ammonia
- H2N2 molecule.
| 2H2O + O2 + 4NH3 → 4H2O excludes 2NH3 excludes H2N2 |
Molecule content in (Figure 69)
| Figure 69 | Molecule content | ||||||
 |
 |
 |
 |
 |
 |
 |
|
| H2O | H2O | H2O | H2O | NH3 | NH3 | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| 4H2O + 2NH3 + H2N2 → H16N4O4 → 4H4NO |
NEW SINGULARITY BEGINNING
| Molecule content | |||
 |
 |
 |
|
| H2O | H2O | H2N2 | |
| H2O + H2O + C → CH4O2 + H2N2 → (NH2)2CO excludes H2O | See Creation of Urea |
| Molecule content | ||||
 |
 |
 |
 |
|
| H2O | H2O | NH3 | NH3 | |
| H2O + H2O + C → CH4O2 + 2NH3 → (NH2)2CO excludes H2O excludes H4 | See Creation of Urea |
| excluded 2H2O + C → CH4O2 |
| excluded H4 + C → CH4 |
CONCLUSION
A singularity is a chemical evolution of Carbon atoms, that is an increasing number of mutually connected C atoms , from an initial C form, over transforming C3,C3 or 2C2 forms to the last C4 form.
The forms and chemistry elements are different for same Creatinin molecule. (see Figure 70, 71, 72)
Figure 70
| C4H7N3O molecule content | ||||||||
| C | CH4 | NH3 | CO | CN2 | See C Creatinin molecule | |||
| C2 | CH4 | NH3 | CN2O | See C2 Creatinin molecule | ||||
| C3 | CH4 | NH3 | N2O | See C3 Creatinin molecule | ||||
| C3 | CH4 | H2O | HN3 | See C3 Creatinin molecule | ||||
| 2C2 | NH3 | H2O | H2N2 | See 2C2 Creatinin molecule | ||||
| C4 | NH3 | H2O | H2N2 | See C4 Creatinin molecule | ||||
| C4 | NH3 | H2O | H2N2 | See C4 Creatinin molecule. | ||||
Figure 71
| C4H8N4 molecule content | ||||||||
| C | 2CH4 | 2CH4 | CN4 | See C Creatinin molecule. | ||||
| C2 | 2CH4 | N4 | See C2 Creatinin molecule. | |||||
| C3 | CH4 | 2H2N2 | See C3 Creatinin molecule. | |||||
| C3 | CH4 | NH3 | HN3 | See C3 Creatinin molecule. | ||||
| C3 | 2NH3 | CH2N2 | See C3 Creatinin molecule. | |||||
| 2C2 | 4NH2 | See 2C2 Creatinin molecule. | ||||||
| 2C2 | 2NH3 | H2N2 | See 2C2 Creatinin molecule. | |||||
| C4 | 4NH2 | See C4 Creatinin molecule. | ||||||
| C4 | 2NH3 | H2N2 | See C4 Creatinin molecule. | |||||
Figure 72
| C4H4O4 molecule content | ||||||||
| C | CH4 | CO2 | CO2 | See C Creatinin molecule. | ||||
| C2 | CH4 | CO2 | O2 | See C2 Creatinin molecule. | ||||
| C3 | CO2 | 2H2O | See C3 Creatinin molecule. | |||||
| 2C2 | O2 | 2H2O | See 2C2 Creatinin molecule. | |||||
| C4 | O2 | 2H2O | See C4 Creatinin molecule. | |||||
| Different molecules forms and content demonstrate the evolution of the Creatinin molecule. |
Author
Vladimir Lazic Denmark September 2012
----------------------------------------------------------------------------------------------------------------------------------------
Part five
ANALYTICAL PROCESS FOR A SINGULAR AND BINARY UREA MOLECULE (NH2)2CO
See Singular and Binary table
See Oxocarbon
See Analytical chemistry
One Urea molecule disintegrates in:
| - C atom See Carbon | C | 1 atom | 1. singularity |
| - N2 molecule See Nitrogen | N | 2 atoms | 2. singularity |
| - H4molecule See Hydrogen | H | 4 atoms | 4. singularity |
| - O atom See Oxygen | O | 1 atom | 8. singularity |
| Oxygen atom is a result of equality in singularity eight. Equality of (eight protons, eight neutrons, eight electrons (2 and 6) See Oxygen |
| Figure 71 | Molecule content | |||
 |
||||
| C | 2N | 2H2 | O | |
Example 14 |
Monoxide Urea molecule
See Carbon monoxide
See S Solidification process
Chemical multiple synthesis: See Chemical synthesis
| H2N + H2N + CO → (NH2)2CO |
| (NH2)2CO |
Urea molecule disintegrates in:
- C atom See Carbon
- H2O molecule See Water
- H2N2 molecule See Figure 72
| C excludes H2O excludes H2N2 |
Urea molecule content with C atom in Figure 72
| Figure 72 | Molecule content | |||
 |
||||
| C | H2O | H2N2 | ||
Chemical multiple synthesis: See Chemical synthesis
| C + H2O + H2N2 → (NH2)2CO |
C Urea molecule in Figure 73
| Figure 73 |
Example 15 |
Chemical multiple synthesis of Urea molecule (NH2)2CO
Chemical multiple synthesis is: See Chemical synthesis
Multiple chemical transformations of Urea molecules.
Urea molecule + Urea molecule → Two Urea molecules in One C2 molecule.
| (NH2)2CO + (NH2)2CO → 2(NH2)2CO |
Two Urea molecules
| 2(NH2)2CO |
Two Urea molecule disintegrate in:
- C2 molecule See Carbon
- 2H2O molecules See Water
- 2H2N2 molecules See Figure 74
| C 2 excludes 2H2O excludes 2H2N2 |
C2 Urea molecule content in Figure 74
| Figure 74 | Molecule content | ||||||
 |
 |
||||||
| C2 | H2O | H2O | H2N2 | H2N2 | |||
Chemical multiple synthesis: See Chemical synthesis
| C2 + 2H2O + 2H2N2 → 2(NH2)2CO |
C2 molecule (Two Urea) See Figure 75
| Figure 75 |
Example 16 |
Chemical multiple synthesis of Urea molecule (NH2)2CO
Multiple chemical transformations of Urea molecules.
Two Urea molecules + Urea molecule → three Urea molecules in one C3 molecule.
| 2(NH2)2CO + (NH2)2CO → 3(NH2)2CO |
Three Urea molecules
| 3(NH2)2CO |
Three Urea molecules disintegrate in:
- C3 molecule See Carbon
- 3H2O molecules See Water
- 3H2N2 molecules See Figure 76
| C 3 excludes 3H2O excludes 3H2N2 |
C3 Urea molecule content in Figure 76
| Figure 76 | Molecule content | ||||||
 |
 |
 |
|||||
| C3 | H2O | H2O | H2O | H2N2 | H2N2 | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C3 + 3H2O + 3H2N2 → 3(NH2)2CO |
C3 molecule (Three Urea) See Figure 77
| Figure 77 |
Example 17 |
Chemical multiple synthesis of Urea molecule (NH2)2CO
Multiple chemical transformations of Urea molecules.
Three Urea molecules + Urea molecule → four Urea molecules in one C4 molecule.
| 3(NH2)2CO + (NH2)2CO → 4(NH2)2CO |
Four Urea molecules
| 4(NH2)2CO |
Four Urea molecules disintegrate in:
- C4 molecule See Carbon
- 4H2O molecules See Water
- 4H2N2 molecules See Figure 78
| C 4 excludes 4H2O excludes 4H2N2 |
C4 Urea molecule content in Figure 78
| Figure 78 | Molecule content | ||||||||
 |
 |
 |
 |
||||||
| C4 | H2O | H2O | H2O | H2O | H2N2 | H2N2 | H2N2 | H2N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C4 + 4H2O + 4H2N2 → 4(NH2)2CO |
C4 molecule (Four Urea) See Figure 79
| Figure 79 |
Example 18 |
Chemical transformations of Urea molecule 4(NH2)2CO in Equal Creatinin molecule 4CNHO
| 4(NH2)2CO → 4CNHO excludes 4NH3 See Ammonia |
| CNH | 2CNH | 3CNH | 4CNH | |||||||||
| O | O2 | O3 | O4 | |||||||||
| CNHO | 2CNHO | 3CNHO | 4CNHO | → | ||||||||
| NH3 | 2NH3 | 3NH3 | 4NH3 | |||||||||
| (NH2)2CO | → | 2(NH2)2CO | → | 3(NH2)2CO | → | 4(NH2)2CO |
Example 19 |
Chemical transformations of Urea molecule 4(NH2)2CO in Equal Creatinin molecule 4CNH
| 4(NH2)2CO → 4CNH excludes 4NH3 See Ammonia excludes 2O2 See Oxygen |
| 3C | CH4 | 4N | |
||
| 4CNH | → | ||||
| 2O2 | |||||
| 4CNHO | |
||||
| 4NH3 | |||||
| 4(NH2)2CO | |||||
Example 20 |
Chemical transformations of Urea molecule 4(NH2)2CO in Equal Creatinin molecule 4COH
| 4(NH2)2CO → 4COH excludes 4NH3 See Ammonia excludes 4N See Nitrogen |
| 3C | CH4 | 2O2 | |
||
| 4COH | → | ||||
| 4N | |||||
| 4CNHO | |
||||
| 4NH3 | |||||
| 4(NH2)2CO | |||||
Example 21 |
Chemical transformations of Urea molecule 4(NH2)2CO in Singular Creatinin molecule C4N8H8 (4(NH)2C)
| 4(NH2)2CO → C4N8H8 excludes 4H2O See Water |
| 2NH | 4NH | 8NH | |||||
| C | 2C | 4C | |||||
| (NH)2C | 2(NH)2C | 4(NH)2C | → | ||||
| H2O | 2H2O | 4H2O | |
||||
| (NH2)2CO | 2(NH2)2CO | 4(NH2)2CO |
Example 21A |
Chemical transformations of Urea molecule 4(NH2)2C in Equal Creatinin molecule 8NH
| 4(NH2)2C → 8NH excludes 4C See Carbon |
| 2NH | 4NH | 8NH | → | ||||
| C | 2C | 4C | |||||
| (NH)2C | 2(NH)2C | 4(NH)2C | |||||
| H2O | 2H2O | 4H2O | |
||||
| (NH2)2CO | 2(NH2)2CO | 4(NH2)2CO |
Example 21B |
Chemical transformations of Urea molecule 4(NH2)2C in Singular Creatinin molecule C4N4H8
| 4(NH2)2C → C4N4H8 excludes 4N See Nitrogen |
| CNH2 | 2CNH2 | 4CNH2 | → | ||||
| N | 2N | 4N | |||||
| (NH)2C | 2(NH)2C | 4(NH)2C | |||||
| H2O | 2H2O | 4H2O | |
||||
| (NH2)2CO | 2(NH2)2CO | 4(NH2)2CO |
Example 22 |
Chemical transformations of Urea molecule 4(NH2)2CO in Singular molecule N8O4
| 4(NH2)2CO → N8O4 excludes 4CH4 See Methane |
| O | O2 | 2O2 | |||||
| N2 | N4 | N8 | |||||
| N2O | N4O2 | N8O4 | → | ||||
| CH4 | 2CH4 | 4CH4 | |
||||
| (NH2)2CO | 2(NH2)2CO | 4(NH2)2CO |
Example 22A |
Chemical transformations of molecule N8O4 in molecule N8
| N8O4 → N8 See Nitrogen excludes O4 See Oxygen |
| O | O2 | 2O2 | |||||
| N2 | N4 | N8 | → | ||||
| N2O | N4O2 | N8O4 | |||||
| CH4 | 2CH4 | 4CH4 | |
||||
| (NH2)2CO | 2(NH2)2CO | 4(NH2)2CO |
Example 22B |
Creation of di-monoxide Urea molecule (NH2)2CO excludes O
See Figure 80
See Carbon dioxide
See S Solidification process
See Creation of two Urea molecules.
Chemical multiple synthesis: See Chemical synthesis
| H2N + H2N + CO2 → (NH2)2CO excludes O See Oxygen |
Urea molecule content with C atom and excluded O in Figure 80
| Figure 80 | (NH2)2CO molecule | excluded O | ||
 |
 |
|||
| C | H2O | H2N2 | O | |
Transformation H2N2 in dihydrogen monoxide (H2O)
Oxygen atom + H2N2 molecule → H2O molecule excludes N2 molecule. See Figure 80A
| O + H2N2 → H2O excludes N2 |
| Figure 80A |  |
di-monoxide Urea molecule content has two water molecules |
Creation of Carbon dioxide Urea molecule (NH2)2CO2
See Figure 81See Carbon dioxide
See S Solidification process
Chemical multiple synthesis: See Chemical synthesis
Two H2N molecules + CO2 molecule → (NH2)2CO2 Carbon dioxide Urea molecule. See Figure 81
| H2N + H2N + CO2 → (NH2)2CO2 |
| Figure 81 | 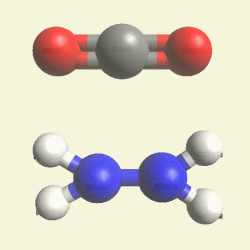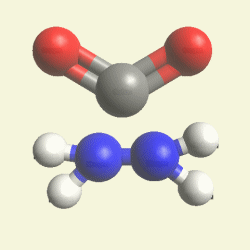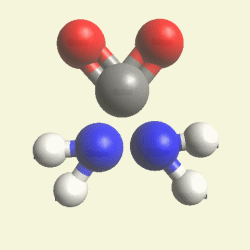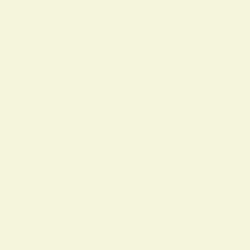 |
Dioxide Urea molecule content has two water molecules |
Example 23 |
Dioxide Urea molecule
| (NH2)2CO2 |
Dioxide Urea molecule disintegrates in:
- C atom See Carbon
- 2H2O molecules See Water
- N2 molecule See Nitrogen
| C excludes 2H2O excludes N2 |
Dioxide Urea molecule content with C atom in Figure 82
| Figure 82 | Molecule content | |||
| C | H2O | H2O | N2 | |
Chemical multiple synthesis: See Chemical synthesis
| C + H2O + H2O + N2 → (NH2)2CO2 |
C Dioxide Urea molecule in Figure 83
| Figure 83 |
Example 24 |
Chemical multiple synthesis of carbon dioxide Urea molecule (NH2)2CO2
Chemical multiple synthesis is: See Chemical synthesis
Multiple chemical transformations of carbon dioxide Urea molecules.
dioxide Urea molecule + dioxide Urea molecule → Two dioxide Urea molecules in one C2 molecule.
| (NH2)2CO2 + (NH2)2CO2 → 2(NH2)2CO2 |
Two dioxide Urea molecules
| 2(NH2)2CO2 |
Two dioxide Urea molecules disintegrate in:
- C2 molecule See Carbon
- 4H2O molecules See Water
- 2N2 molecules See Figure 84
| C 2 excludes 4H2O excludes 2N2 |
Two dioxide Urea molecule content with C2 atoms in Figure 84
| Figure 84 | Molecule content | ||||||
| C2 | H2O | H2O | H2O | H2O | 2N2 | ||
Chemical multiple synthesis: See Chemical synthesis
| C2 + 4H2O + 2N2 → 2(NH2)2CO2 |
C2 molecule (Two dioxide Urea) See Figure 85
| Figure 85 |
Example 25 |
Chemical multiple synthesis of carbon dioxide Urea molecule (NH2)2CO2
Multiple chemical transformations of carbon dioxide Urea molecules.
Two dioxide Urea molecules + dioxide Urea molecule → three dioxide Urea molecules in one C3 molecule.
| 2(NH2)2CO2 + (NH2)2CO2 → 3(NH2)2CO2 |
Three dioxide Urea molecules
| 3(NH2)2CO2 |
Three dioxide Urea molecules disintegrate in:
- C3 molecule See Carbon
- 6H2O molecules See Water
- N6 molecule (See figure 86)
| C 3 excludes 6H2O excludes N6 molecule See Nitrogen |
C3 Urea molecule content in Figure 86
| Figure 86 | Molecule content | |||||||
| C3 | H2O | H2O | H2O | H2O | H2O | H2O | N6 | |
Chemical multiple synthesis: See Chemical synthesis
| C3 + 6H2O + N6 → 3(NH2)2CO2 |
C3 Urea molecule See Figure 87
| Figure 87 |
Example 26 |
Chemical multiple synthesis of carbon dioxide Urea molecule (NH2)2CO2
Multiple chemical transformations of Carbon dioxide Urea molecules.
Three dioxide Urea molecules + dioxide Urea molecule → four dioxide Urea molecules in one C4 molecule.
| 3(NH2)2CO2 + (NH2)2CO2 → 4(NH2)2CO2 |
Four dioxide Urea molecules
| 4(NH2)2CO2 |
Four dioxide Urea molecules disintegrate in:
- C4 molecule See Carbon
- 8H2O molecules See Water
- N8 molecule See Figure 88
| C 4 excludes 8H2O excludes N8 See Nitrogen |
C4 Urea molecule content in Figure 88
| Molecule content | |||||||||
| C4 | H2O | H2O | H2O | H2O | H2O | H2O | H2O | H2O | N8 |
Chemical multiple synthesis: See Chemical synthesis
| C4 + 8H2O + N8 → 4(NH2)2CO2 |
C4 Urea molecule See Figure 89
| Figure 89 |
Example 27 |
Molecule division
Molecule division is the process by which a four Urea molecule divides into 2C2 molecules See Figure 90
Division is a small part of a larger chemical process. This process is known as binary increasing (C2 - C3 - 2C2 - C4), by which molecules divide again.
The molecule is permanently transformed into these chemistry cicle Urea - Creatinin - Urea molecule.
| Figure 90 | 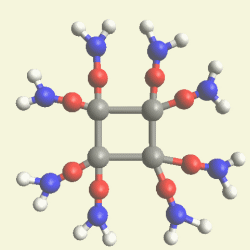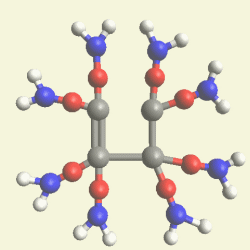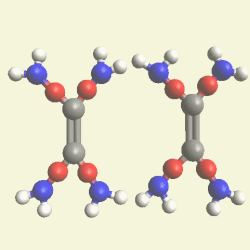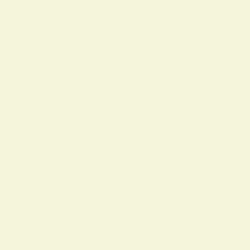 |
Example 28 |
Chemical multiple synthesis of Methane Urea molecule (NH2)2CO
See Methane
See Nitrogen
See Hydrogen
Chemical transformation CH4 and N2O molecule See Example 2A
CH4 molecule + N2O molecule → Methan Urea molecule See Figure 91
| CH4 + N2O → (NH2)2CO |
| Figure 91 | Molecule content | |||
 |
||||
| CH4 | N2O | |||
Methan Urea molecule (NH2)2CO2
| CH4 + N2O2 → (NH2)2CO2 |
Example 29 |
Chemical multiple synthesis of Ammonia Urea molecule (NH2)2CO
See Ammonia
See Carbon
See Nitrogen
See Hydrogen
CNHO molecule See Example 18
NH3 molecule + CNHO molecule → Ammonia Urea molecule See Figure 92
| NH3 + CNHO → (NH2)2CO |
| Figure 92 | Molecule content | |||
 |
||||
| NH3 | CHNO | |||
Ammonia Urea molecule (NH2)2CO2
| NH3 + CNHO2 → (NH2)2CO2 |
Example 30 |
Chemical multiple synthesis of Water Urea molecule (NH2)2CO2
See Water
See Carbon
See Nitrogen
Two water molecule + CN2 molecule → Water Urea molecule See Figure 93
| 2H2O + CN2 → (NH2)2CO2 |
| Figure 93 | Molecule content | |||
| H2O | H2O | CN2 | ||
Example 31 |
Monoxide Urea - Creatinin molecule
See Carbon monoxide
See S Solidification process
Chemical multiple synthesis: See Chemical synthesis
Two H2N molecules + Carbon monoxide molecule → Monoxide Urea molecule.
| 2H2N + CO → (NH2)2CO |
Four H2N molecules + two Carbon monoxide molecules → two Monoxide Urea molecules.
| 4H2N + 2CO → 2(NH2)2CO |
Four H2N molecules + Carbon monoxide molecule → Monoxide Creatinin molecule.
| 4H2N + CO → CH8N4O |
Monoxide Creatinin molecule CH8N4O
See Figure 101
| Figure 101 |
Example 32 |
Dioxide Urea - Creatinin molecule
See Carbon dioxide
See S Solidification process
Chemical multiple synthesis: See Chemical synthesis
Two H2N molecules + Carbon dioxide molecule → Dioxide Urea molecule.
| 2H2N + CO2 → (NH2)2CO2 |
Four H2N molecules + two Carbon dioxide molecules → two Dioxide Urea molecules.
| 4H2N + 2CO2 → 2(NH2)2CO2 |
Four H2N molecules + Carbon dioxide molecule → Dioxide Creatinin molecule.
| 4H2N + CO2 → CH8N4O2 |
Dioxide Creatinin molecule CH8N4O2
See Figure 102
| Figure 102 |
CARBON ATOMS REDUCTION PROCESS FOR A SINGULAR CREATININ MOLECULE C4H8N4
Example 33 |
See S Solidification process
See Carbon
Singular Creatinin molecule C4H8N4 become CH8N4 molecule exclludes C3 molecule.
| C4H8N4 → CH8N4 excludes C3 |
One Carbon atom creating molecule in chemical synthesis:
| 4H2N + C → CH8N4 |
Creatinin molecule CH8N4 See Figure 103
| Figure 103 |
Singular Creatinin molecule C4H8N4 become C2H8N4 molecule excludes C2 molecule.
See Carbon
| C4H8N4 → C2H8N4 excludes C2 |
Two Carbon atoms creating molecule in chemical synthesis:
| 4H2N + C2 → C2H8N4 |
Creatinin molecule C2H8N4 See Figure 104
| Figure 104 |
Creatinin molecule C4H8N4 becomes C3H8N4 molecule excludes C atom.
See Carbon
| C4H8N4 → C3H8N4 excludes C |
Three Carbon atoms creating molecule in chemical synthesis:
| C3 + 4H2N → C3H8N4 |
Creatinin molecule C3H8N4 See Figure 105
| Figure 105 | 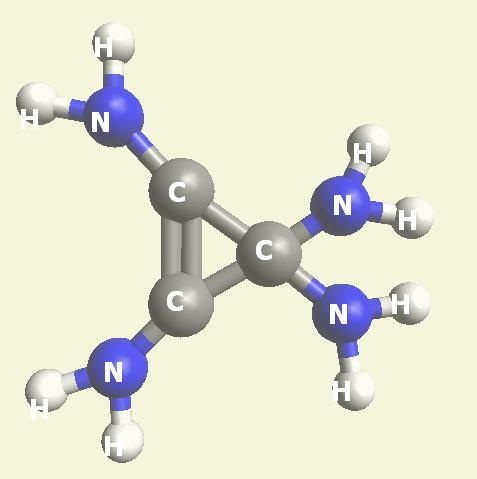
|
Example 34 |
CARBON ATOMS REDUCTION PROCESS FOR A CREATININ MOLECULE C4H7N3O
See Solidification process for Creatinin molecule
See Carbon
Creatinin molecule C4H7N3O become CH7N3O molecule excludes C3 molecule.
| C4H7N3O → CH7N3O excludes C3 |
One Carbon atom creating molecule in chemical synthesis:
| CO + H7N3 → CH7N3O |
Creatinin molecule CH7N3O See Figure 106
| Figure 106 | 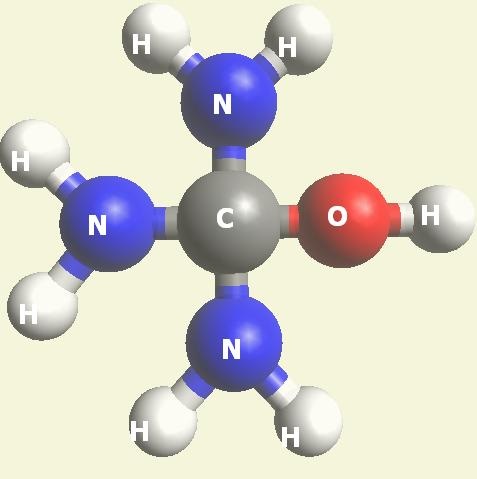
|
Creatinin molecule C4H7N3O become C2H7N3O molecule and excludes C2 molecule.
See Carbon
| C4H7N3O → C2H7N3O excludes C2 |
Two Carbon atoms creating a molecule in chemical synthesis:
| C2 + H7N3O → C2H7N3O |
Creatinin molecule C2H7N3O See Figure 107
| Figure 107 | 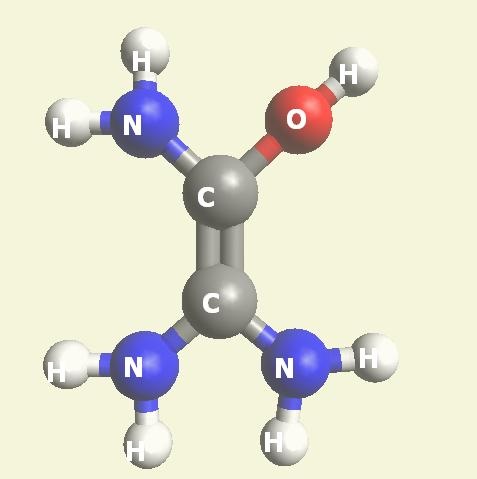
|
Creatinin molecule C4H7N3O becomes C3H7N3O molecule excludes C atom.
See Carbon
| C4H7N3O → C3H7N3O excludes C |
Three Carbon atoms creating molecule in chemical synthesis:
| C3 + H7N3O → C3H7N3O |
Creatinin molecule C3H7N3O See Figure 108
| Figure 108 | 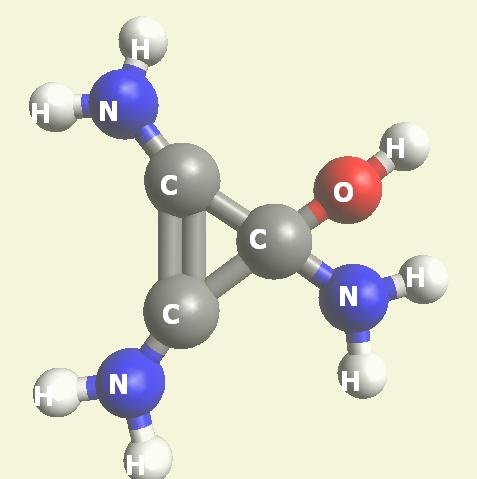
|
Example 35 |
CARBON ATOMS REDUCTION PROCESS FOR A CREATININ MOLECULE C4H4O4
See Solidification process for Creatinin molecule
See Carbon
Creatinin molecule C4H4O4 becomes CH4O4 molecule excludes C3 molecule.
| C4H4O4 → CH4O4 excludes C3 |
One Carbon atom creating molecule in chemical synthesis:
| C + 2H2O2 → CH4O4 |
Creatinin molecule CH4O4 See Figure 109
| Figure 109 | 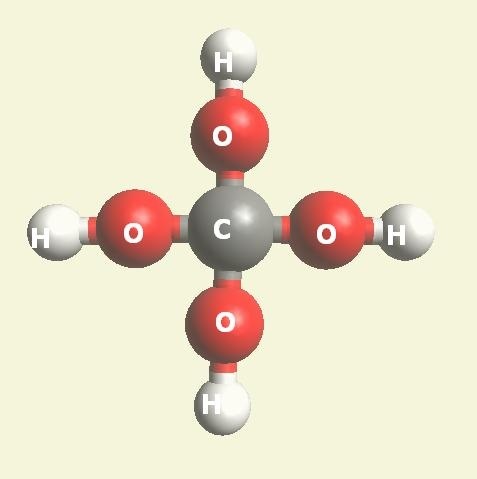
|
E Creatinin molecule C4H4O4 become C2H4O4 molecule excludes C2 molecule.
See Carbon
| C4H4O4 → C2H4O4 excludes C2 |
Two Carbon atoms creating a molecule in chemical synthesis:
| C2 + 2H2O2 → C2H4O4 |
Creatinin molecule C2H4O4 See Figure 110
| Figure 110 | 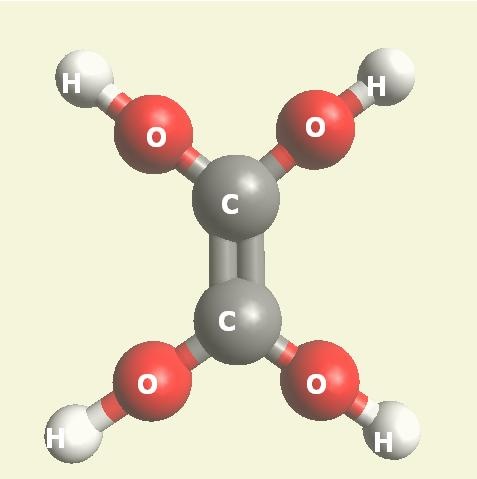
|
Creatinin molecule C4H4O4 becomes C3H4O4 molecule excludes C atom.
See Carbon
| C4H4O4 → C3H4O4 excludes C |
Three Carbon atoms creating a molecule in chemical synthesis:
| C3 + 2H2O2 → C3H4O4 |
Creatinin molecule C3H4O4 See Figure 111
| Figure 111 |
Example 36 |
UREA ZERO-POINT (Urea without Carbon and Oxygen atoms)
Carbon and Oxygen atoms do not influence chemical transformation, and theoretically can exclude from Urea molecule.
Creating 2NH2 molecule in chemical transformation. (See Figure 112)
Urea molecule → (NH2)2O molecule excludes Carbon atom.
| (NH2)2CO → (NH2)2O excludes C |
(NH2)2O molecule → (NH2)2 molecule excludes Oxygen atom.
| (NH2)2O → (NH2)2 excludes O |
Excluded C + O → CO See Carbon monoxide
| Figure 112 | 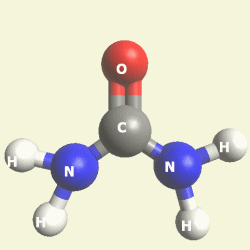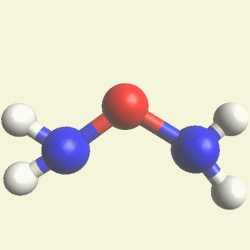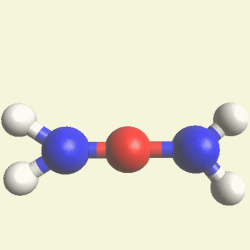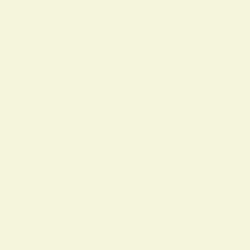
|
Chemical transformation of (NH2)2 molecule in NH3O molecule
(See Figure 113)
(NH2)2 molecule + water molecule → NH3O molecule excludes Ammonia molecule.
| (NH2)2 + H2O → NH3O excludes NH3 See Ammonia |
| Figure 113 |
(NH2)2 molecule + two water molecules → Hydrogen peroxide molecule excludes two Ammonia molecules.
| (NH2)2 + 2H2O → H2O2 excludes 2NH3 See Ammonia |
Hydrogen peroxide molecule See Figure 114
| Figure 114 | 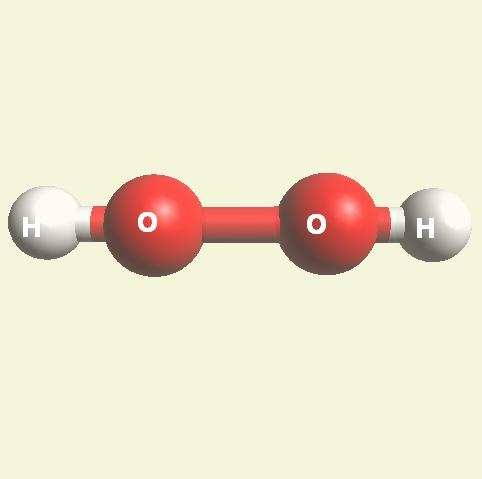
|
See Hydrogen peroxide
See Water
See Oxygen
Hydrogen peroxide molecule → water molecule excludes Oxygen atom.
| H2O2 → H2O excludes O See Oxygen |
Chemical transformation of (NH2)2 molecule in 2N molecule
See Figure 115
(NH2)2 molecule + Carbon atom → 2N molecule excludes Methane molecule.
| (NH2)2 + C → 2N excludes CH4 See Methane |
(NH2)2 molecule + O2 molecule → N2 molecule excludes two Water molecules.
| (NH2)2 + O2 → 2N excludes 2H2O See Water |
2N molecule See Figure 115
| Figure 115 |
Example 37 |
CREATININ ZERO-POINT (Creatinin without Carbon atom)
Chemical process is the transformation of H2O in NH3, without Carbon atom See Figure 116
Creating a molecule in chemical synthesis:
Four Nitrogen atoms + four water molecules → 4NH2 molecules See S Solidification process
| 4N + 4H2O → 4NH2 excludes 2O2 |
4NH2 molecules + two water molecules → four NH3 molecule See Ammonia
| 4NH2 + 2H2O → 4NH3 excludes O2 |
| Figure 116 | .gif)
|
Chemical synthesis:
Four Nitrogen atoms + six water molecules → 4NH3 molecules excludes 3O2
| 4N + 6H2O → 4NH3 excludes 3O2 |
| CH8N4 Creatinin molecule |
See Figure 117
| CH8N4 → 4NH2 excludes C |
| Figure 117 | 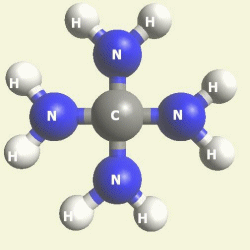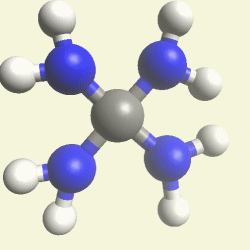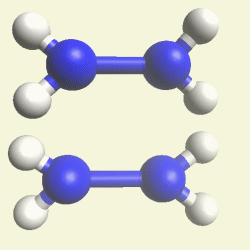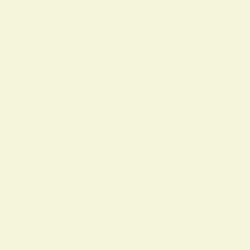
|
| CH7N3O Creatinin molecule |
See Figure 118
Creatinin molecule → 2NH2molecule + NOH3 molecule excludes Carbon atom.
| CH7N3O → 2NH2 + NOH3 excludes C See Carbon |
| Figure 118 | 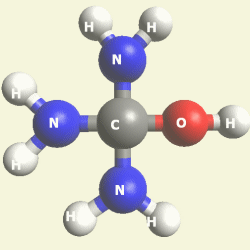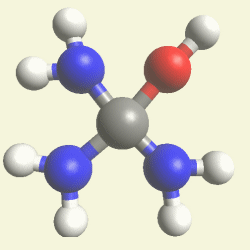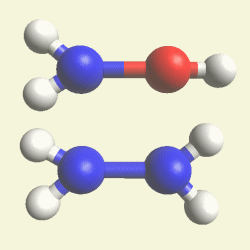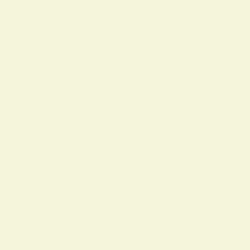
|
NOH3 molecule + NH3 molecule → 2NH2 molecule excludes H2O molecule.
| NOH3 + NH3 → 2NH2 excludes H2O See Water |
2NH2 molecule + H2O molecule → NOH3 molecule excludes NH3 molecule.
| 2NH2 + H2O → NOH3 excludes NH3 See Ammonia |
| CH4O4 Creatinin molecule |
See Figure 119
Creatinin molecule → 2H2O2 molecules excludes Carbon atom.
| CH4O4 → 2H2O2 excludes C See Carbon |
| Figure 119 | 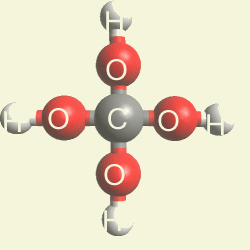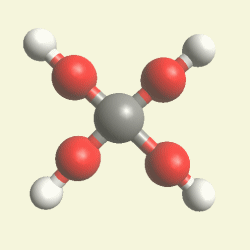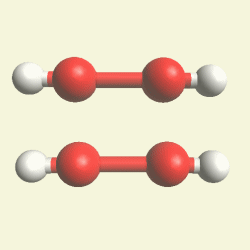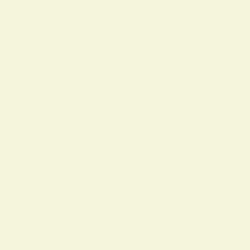
|
See Hydrogen peroxide
See Water
See Oxygen
2H2O2 molecules → 2H2O molecules excludes O2 molecule.
| 2H2O2 → 2H2O excludes O2 |
REPEATING CREATININ FORMS C-C2-C3-C4
Example 38 |
Singularity five
See Singular and Binary table
See Oxocarbon
- Example :
Creates one Carbon atom Creatinin molecule excludes four Carbon molecule :
Creatinin molecule + Carbon atom → Creatinin molecule singularity one, excludes C4 molecule singularity four
| C4H8N4 + C → CH8N4 excludes C4 |
Singular transformation of C4H8N4 molecule in CH8N4 molecule in Figure 120
| Figure 120 | C4H8N4 | → | CH8N4 | excluded |
 |
→ | |||
| C4 | → | C | C4 |
| C4H7N3O + C → CH7N3O excludes C4 |
Singular transformation C4H7N3O molecule in CH7N3O molecule in Figure 121
| Figure 121 | C4H7N3O | → | CH7N3O | excluded |
 |
→ | 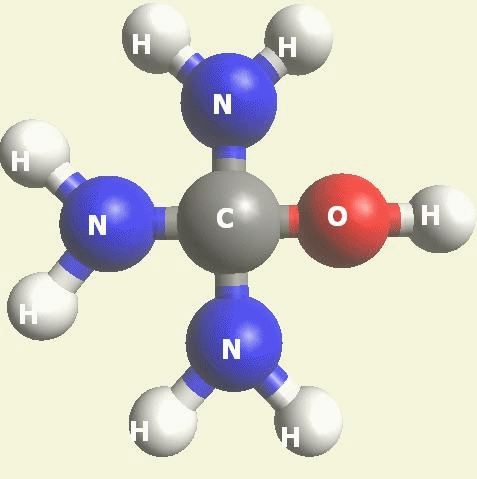 |
||
| C4 | → | C | C4 |
| C4H4O4 + C → CH4O4 excludes C4 |
Singular transformation of C4H4O4 molecule in CH4O4 molecule in Figure 122
| Figure 122 | C4H4O4 | → | CH4O4 | excluded |
| → | 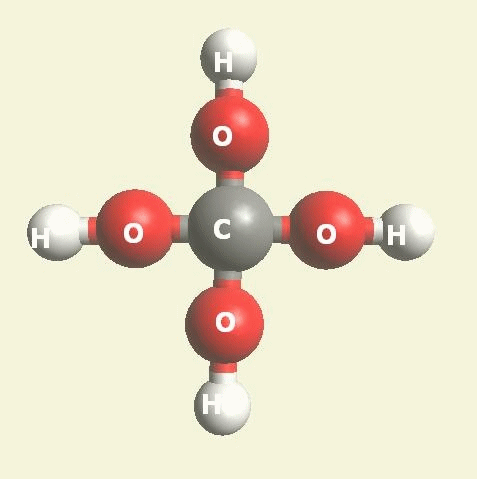 |
|||
| C4 | → | C | C4 |
Example 39 |
Singularity six
See Singular and Binary table
See Oxocarbon
- Example :
Creates two Carbon atoms Creatinin molecule, excludes four Carbon atoms :
Creatinin molecule + two Carbon atoms → Creatinin molecule singularity two, excludes C4 molecule singularity four
| C4H8N4 + C2 → C2H8N4 excludes C4 |
C2H8N4 molecule in Figure 123
| Figure 123 | C2H8N4 | excluded |
| C2 | C4 |
| C4H7N3O + C2 → C2H7N3O excludes C4 |
C2H7N3O molecule in Figure 124
| Figure 124 | C2H7N3O | excluded |
 |
||
| C2 | C4 |
| C4H4O4 + C2 → C2H4O4 excludes C4 |
Singular transformation C4H4O4 molecule in C2H4O4 molecule in Figure 125
| Figure 125 | C2H4O4 | excluded |
 |
||
| C2 | C4 |
Example 40 |
Singularity seven
See Singular and Binary table
See Oxocarbon
- Example :
Creates three Carbon atoms Creatinin molecule, excludes C4 molecule :
Creatinin molecule + three Carbon atoms → Creatinin molecule singularity three, excludes C4 atoms singularity four
| C4H8N4 + C3 → C3H8N4 excludes C4 |
C3H8N4 molecule in Figure 126
| Figure 126 | C3H8N4 | excluded |
 |
||
| C3 | C4 |
| C4H7N3O + C3 → C3H7N3O excludes C4 |
C3H7N3O molecule in Figure 127
| Figure 127 | C3H7N3O | excluded |
 |
||
| C3 | C4 |
| C4H4O4 + C3 → C3H4O4 excludes C4 |
C3H4O4 molecule in Figure 128
| Figure 128 | C3H4O4 | excluded |
| C3 | C4 |
Example 41 |
Singularity eight
See Singular and Binary table
See Oxocarbon
- Example :
Creates four Carbon atoms Creatinin molecule, excludes C4 molecule :
Creatinin molecule + four Carbon atoms → Creatinin molecule singularity four, excludes C4 molecule singularity four
| C4H8N4 + C4 → C4H8N4 excludes C4 |
Singular transformation C4H8N4 molecule in C4H8N4 molecule in Figure 129
| Figure 129 | C4H8N4 | excluded |
| C4 | C4 |
| C4H7N3O + C4 → C4H7N3O excludes C4 |
Singular C4H7N3O molecule in Figure 130
| Figure 130 | C4H7N3O | excluded |
 |
||
| C4 | C4 |
| C4H4O4 + C4 → CH4O4 excludes C4 |
Singular C4H4O4 molecule in Figure 131
| Figure 131 | C4H4O4 | excluded |
| C4 | C4 |
Example 42 |
Singularity nine
See Singular and Binary table)
See Oxocarbon
- Example :
Creates one Carbon atom Creatinin molecule, excludes eight Carbon molecule :
One Carbon atom Creatinin molecule + eight Carbon atoms → Creatinin molecule singularity one, excludes C8 molecule singularity eight
| CH8N4 + C8 → CH8N4 excludes C8 |
Singular CH8N4 molecule in Figure 132
| Figure 132 | <CH8N4 | excluded |
| C | C8 |
| CH7N3O + C8 → CH7N3O excludes C8 |
Singular CH7N3O molecule in Figure 133
| Figure 133 | CH7N3O | excluded |
 |
||
| C | C8 |
| CH4O4 + C8 → CH4O4 excludes C8 |
Singular CH4O4 molecule in Figure 134
| Figure 134 | CH4O4 | excluded |
 |
||
| C | C8 |
Example 43 |
Singularity ten
See Singular and Binary table
See Oxocarbon
- Example :
Creates two Carbon atoms, Creatinin molecule excludes eight Carbon molecule :
Two Carbon atom Creatinin molecule + eight Carbon atoms → Creatinin molecule singularity two excludes C8 molecule singularity eight
| C2H8N4 + C8 → C2H8N4 excludes C8 |
C2H8N4 molecule in Figure 135
| Figure 135 | C2H8N4 | excluded |
| C2 | C8 |
| C2H7N3O + C8 → C2H7N3O excludes C8 |
C2H7N3O molecule in Figure 136
| Figure 136 | C2H7N3O | excluded |
 |
||
| C2 | C8 |
| C2H4O4 + C8 → C2H4O4 excludes C8 |
C2H4O4 molecule in Figure 137
| Figure 137 | C2H4O4 | excluded |
 |
||
| C2 | C8 |
INCREASING CREATININ FORMS C5 → ∞
Carbon atoms singularity four + Carbon atoms singularity one → Carbon atoms singularity five
| C4 + C → C5 |
Example 44 |
Singularity five
See Singular and Binary table
See Oxocarbon
- Example :
Carbon atoms creating a molecule in chemical synthesis:
Singular Creatinin molecule + Carbon atom → C5 Creatinin molecule.
| C4H8N4 + C → C5H8N4 |
C5 Creatinin molecule in Figure 138
| Figure 138 |
Creatinin molecule + Carbon atom → C5 Creatinin molecule.
| C4H7N3O + C → C5H7N3O |
C5 Creatinin molecule in Figure 139
| Figure 139 |
Equal Creatinin molecule + Carbon atom → C5 Creatinin molecule.
| C4H4O4 + C → C5H4O4 |
C5 Creatinin molecule in Figure 140
| Figure 140 |
- Example :
Singular Creatinin molecule + Urea molecule → C5 Creatinin molecule excludes Oxygen atom.
| C4H8N4 + (NH2)2CO → C5H12N6 excludes O |
Creatinin molecule + Urea molecule → C5 Creatinin molecule, excludes Oxygen atom.
| C4H7N3O + (NH2)2CO → C5H11N5 excludes O2 |
Equal Creatinin molecule + Urea molecule → C5 Creatinin molecule excludes H3N2molecule.
| C4H4O4 + (NH2)2CO → C5H5O5 excludes H3N2 |
| Figure 141 | C5H12N6 | C5H11N5 | C5H5O5 |
| excludes O | excludes O2 | excludes H3N2 |
Example 45 |
Singularity six
- Example :
Singular Creatinin molecule + two Urea molecule → C6 Creatinin molecule excludes two Oxygen atoms.
| C4H8N4 + 2(NH2)2CO → C6H16N8 excludes O2 |
Creatinin molecule + two Urea molecule → C6 Creatinin molecule excludes three Oxygen atoms.
| C4H7N3O + 2(NH2)2CO → C6H15N7 excludes O3 |
Equal Creatinin molecule + two Urea molecule → C6 Creatinin molecule excludes 2H3N2molecules.
| C4H4O4 + 2(NH2)2CO → C6H6O6 excludes 2H3N2 |
| Figure 142 | C6H16N8 | C6H15N7 | C6H6O6 |
| excludes O2 | excludes O3 | excludes 2H3N2 |
Singularity seven
- Example :
Singular Creatinin molecule + three Urea molecules → C7 Creatinin molecule excludes three Oxygen atoms.
| C4H8N4 + 3(NH2)2CO → C7H20N10 excludes O3 |
Creatinin molecule + three Urea molecules → C7 Creatinin molecule excludes four Oxygen atoms.
| C4H7N3O + 3(NH2)2CO → C7H19N9 excludes 2O2 |
Equal Creatinin molecule + three Urea molecules → C7 Creatinin molecule excludes 2H3N2molecules.
| C4H4O4 + 3(NH2)2CO → C7H7O7 excludes 2H3N2 |
| Figure 143 | C7H20N10 | C7H19N9 | C7H7O7 |
| excludes O3 | excludes 2O2 | excludes 2H3N2 |
Singularity eight - Dioxide Urea example
- Example with 4(NH2)2CO2:
Singular Creatinin molecule + four dioxide Urea molecules → C8 Creatinin molecule
| C4H8N4 + 4(NH2)2CO2 → C8H24N12O8 |
Creatinin molecule + four dioxide Urea molecules → C8 Creatinin molecule
| C4H7N3O + 4(NH2)2CO2 → C8H23N11O9 |
Equal Creatinin molecule + four dioxide Urea molecule → C8 Creatinin molecule excludes 4N2 molecules.
| C4H4O4 + 4(NH2)2CO2 → C8H16O12 excludes 4HN2 |
| Figure 144 | C8H24N12O8 | C8H23N11O9 | C8H16O12 |
| excludes 4HN2 |
Example 46 |
Singularity eight - Two Creatinin molecules example
- Example :
Singular Creatinin molecule + Singular Creatinin molecule → C8 Creatinin molecule
| C4H8N4 + C4H8N4 → C8H16N8 |
Creatinin molecule + Creatinin molecule → C8 Creatinin molecule
| C4H7N3O + C4H7N3O → C8H14N6O2 |
Equal Creatinin molecule + Equal Creatinin molecule → C8 Equal Creatinin molecule
| C4H4O4 + C4H4O4 → C8H8O8 |
| Figure 145 | C8H16N8 | C8H14N6O2 | C8H8O8 |
Example 47 |
Singularity nine -
- Example :
C8 Singular Creatinin molecule + One Urea molecule → C9 Creatinin molecule
| C8H16N8 + (NH2)2CO → C9H20N10 excludes O |
C8 Creatinin molecule + One Urea molecule → C9 Creatinin molecule
| C8H14N6O2 + (NH2)2CO → C9H18N8O3 |
C8 Equal Creatinin molecule + One Urea molecule → C9 Equal Creatinin molecule
| C8H8O8 + (NH2)2CO → C9H12N2O9 |
| Figure 146 | C9H20N10 | C9H18N8O3 | C9H12N2O9 |
Example 48 |
Singularity ten -
- Example :
C9 Singular Creatinin molecule + One Urea molecule → C10 Creatinin molecule
| C9H20N10 + (NH2)2CO → C10H24N12O |
C9 Creatinin molecule + One Urea molecule → C10 Creatinin molecule
| C9H18N8O3+ (NH2)2CO → C10H22N10O4 |
C9 Equal Creatinin molecule + One Urea molecule → C10 Equal Creatinin molecule
| C9H12N2O9 + (NH2)2CO → C10H16N4O10 |
| Figure 147 | C10H24N12O | C10H22N10O4 | C10H16N4O10 |
Ten Urea molecules example :
| 10(NH2)2CO → C10H40N20O10 |
| Figure 148 | C10H40N20O10 |
|
Chemical Formula: C10H40N20O10 Molecular Weight (g/mol): 600,553 Energy (kJ/mol): 70.215,101 Estmated Dipole Moment(D): 1,460 Number of Atoms: 80 Number of Bonds: 80 |
Example 49 |
Twenty Urea molecules + one thousand Creatinin molecules example.
| 20(NH2)2CO + 1000C4H7N3O → C20H80N40O20 + C4000H7000N3000O1000 → |
| → C4020H7080N3040O1020 |
| Standards of Measurement Etalons (sum of atoms): | ||
| singularity | molecule | definitive |
| 1 | C4020H7080N3040O1020 | - in 1 volume (mmol/l) millimoles per liter |
Sum of atoms CHNO in blood is 4020 + 7080 + 3040 + 1020 = 150160 = 100%
| Standards of Measurement Etalons ( % ): | ||
| atom : | % | condition |
| Carbon atoms | 40,2 % | solid |
| Oxygen atoms | 10,2 % | liquid |
| Hydrogen atoms | 20,4 % | |
| Hydrogen atoms | 50,4 % | gas |
| Nitrogen atoms | 30,4 % | gas |
| Standards of Measurement Etalons (atom): | ||
| singularity | atoms | molecule |
| 1 | 1 Oxygen atom | - in 1 Water molecule |
| 1 | 1 Oxygen atom | - in 1 Carbon monoxide molecule |
| 1 | 1 Nitrogen atom | - in 1 Ammonia molecule |
| 1 | 1 Carbon atom | - in 1 Carbon monoxide molecule |
| 1 | 1 Carbon atom | - in 1 Carbon dioxide molecule |
| 1 | 1 Carbon atom | - in 1 Methane molecule |
| 2 | 2 Nitrogen atoms | - in 1 CN2O and CN2 molecule |
| 2 | 2 Hydrogen atoms | - in 1 Water molecule |
| 2 | 2 Oxygen atoms | - in 1 Carbon dioxide molecule |
| 3 | 3 Hydrogen atoms | - in 1 Ammonia molecule |
| 4 | 4 Hydrogen atoms | - in 1 Methane molecule |
| 19 | Total number atoms for Urea-Creatinin Molecules | |
| Standards of Measurement Etalons (atom): | ||
| singularity | singularity | chem elem |
| 1 | 3 | Nitrogen atoms |
| 2 | ||
| Standards of Measurement Etalons (atom): | ||
| singularity | singularity | chem elem |
| 1 | 3 | Carbon atoms |
| 1 | ||
| 1 | ||
| Standards of Measurement Etalons (atom): | ||
| singularity | singularity | chem elem |
| 1 | 4 | Oxygen atoms |
| 1 | ||
| 2 | ||
| Standards of Measurement Etalons (atom): | ||
| singularity | singularity | chem elem |
| 2 | 9 | Hydrogen atoms |
| 3 | ||
| 4 | ||
Singularity create number of Methane molecules
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | Methane molecules |
| 1 | 1005 | Carbon atoms | 1005 |
| 4 | 4020 | Hydrogen atoms | |
Singularity create number of Ammonia molecules
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | Ammonia molecules | |
| 1 | 1020 | Nitrogen atoms | 1020 | |
| 3 | 3039 | Hydrogen atoms | ||
| 2 | 21 | |||
Singularity creates a number of CN2O molecules
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | CN2O molecules |
| 1 | 1005 | Oxygen atoms | 1005 |
| 1 | 1005 | Carbon atoms | |
| 2 | 2010 | Nitrogen atoms | |
Singularity creates a number of CN2 molecules
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | CN2 molecules |
| 1 | 5 | Carbon atoms | 5 |
| 2 | 10 | Nitrogen atoms | |
Singularity creates a number of Carbon monoxide molecules
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | Carbon monoxide molecule |
| 1 | 15 | Oxygen atoms | 15 |
| 1 | 15 | Carbon atoms | |
Singularity creates a number of C2 molecules
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | C2 molecule |
| 1 | 1990 | Carbon atoms | 995 |
Singularity creates a number of missing O2 molecules
Transformation C2 moolecule in 2CO2 molecule.
| Standards of Measurement Etalons (molecule): | |||
| Singularity | atoms | molecules | Carbon dioxide mol. |
| 1 | 1990 | Carbon atoms | 1990 |
| 2 | 3980 | Oxygen atoms | |
Singularity creates a number of missing O2 molecules
Transformation CH4 moolecule in CO2 molecule.
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | Carbon dioxide mol. |
| 1 | 1005 | Carbon atoms | 1005 |
| 2 | 2010 | Oxygen atoms | |
Singularity creates a number of missing O molecule
Transformation CH4 molecule in CO molecule.
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | Carbon monoxide mol. |
| 1 | 1005 | Carbon atoms | 1005 |
| 1 | 1005 | Oxygen atoms | |
Singularity creates a number of H2O molecules
Transformation CH4 molecule in H2O molecule.
| Standards of Measurement Etalons (molecule): | |||
| singularity | atoms | molecules | Water molecule |
| 2 | 2010 | Oxygen atoms | 2010 |
| 4 | 4020 | Hydrogen atoms | |
Singularity creates a number of missing Haemoglobin cell
Transformation of number Nitrogen atoms in FeN4 Haemo molecule core.
Nitrogen atoms number divided by four, for number Haemo molecules
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | Haemo molecules core | |
| 1 | 3040 | Nitrogen atoms | 760 | |
| 4 | 760 | N4 atoms | ||
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | 25% damaged Haemo molecules core | |
| 1 | 3040 | Nitrogen atoms | 253 | |
| 12 | 253 | 4N3 atoms | ||
Nitrogen atoms number divided by sixteen for Haemoglobin cell number.
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | Haemoglobin cell | |
| 1 | 3040 | Nitrogen atoms | 190 | |
| 16 | 190 | 4N4 atoms | ||
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | 25% damaged Haemoglobin cell | |
| 1 | 3040 | Nitrogen atoms | 253 | |
| 12 | 253 | 4N3 atoms | ||
Singularity create number of missing Iron atoms
Transformation of Nitrogen atoms, in Fe core in Haemoglobin cell.
3040/4=760
| Standards of Measurement Etalons (molecule): | ||||
| singularity | atoms | molecules | Haemo molecules Fe core | |
| 1 | 3040 | Nitrogen atoms | 760 | |
| 4 | 760 | 4Fe atoms | ||
Example 50 |
| C4H7N3O molecule content | ||||||||
| C | CH4 | NH3 | CO | CN2 | See C Creatinin molecule | |||
| C2 | CH4 | NH3 | CN2O | See C2 Creatinin molecule | ||||
| C3 | CH4 | NH3 | N2O | See C3 Creatinin molecule | ||||
| C3 | CH4 | H2O | HN3 | See C3 Creatinin molecule | ||||
| 2C2 | NH3 | H2O | H2N2 | See 2C2 Creatinin molecule | ||||
| C4 | NH3 | H2O | H2N2 | See C4 Creatinin molecule | ||||
| C4 | NH3 | H2O | H2N2 | See C4 Creatinin molecule. | ||||
Author
Vladimir Lazic Denmark March 2013
------------------------------------------------------------------------------------------
Part six
NEW SINGULARITY BRANCH (Phosphorus atom as central atom)
PHOSPHATE MOLECULES PO4; HPO4; H2PO4; H3PO4; H4PO4; H5PO5.....
The chemical process can change the content, with the chemical synthesis of the Phosphorus atom.
The Carbon or Phosphorus atoms, as central atoms, determine the kind of molecules.
The beginning of the next singularity is determined by the number of electrons. See Figure 149
Figure 149
| Molecule content | ||||||
| Periphery atoms | Central atoms | |||||
| atom |  |
 |
 |
 |
 |
|
| atom | Hydrogen | Oxygen | Nitrogen | Carbon | Phosphorus | |
| electrons | 1 | 2 - 6 | 2 - 5 | 2 - 4 | 2 - 8 - 5 | |
| mising electrons | 7 | 2 | 3 | 4 | 3 | |
| singularity | 1 | 2 | 3 | 4 | 5 | |
The electron bond is the sum of electron number + missing electrons number = 8.
The singularity number is the distance from the zero point.
ANALYTICAL CHEMISTRY OF THE PHOSPHATE MOLECULE
NEXT |